New Drugs and Therapies: Maui Derm 2019 Highlights
Hana hou! The “Ted and Neal Show” once again entertained and educated attendees about new drugs and therapies in dermatology and how we will be using them in 2019.

Ted Rosen, MD
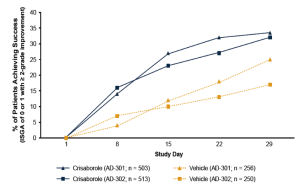
Drs. Rosen and Bhatia discussed halobetasol (HP) for the treatment of psoriasis, including a study comparing HP (0.01%) lotion versus HP cream (0.05%). In addition, they discussed treatment success of halobetasol 0.01%/tazarotene 0.045% (HP/TAZ) versus vehicle in two separate studies. Other studies on HP/TAZ included a Phase 2 comparison of HP/TAZ with halobetasol cream 0.05% and a separate study comparing HP/TAZ and tazarotene cream 0.05%.

Neal Bhatia, MD
Ted and Neal also discussed recent research on cemiplimab for advanced non-melanoma skin cancer (NMSC). Cemiplimab is a human monoclonal antibody directed towards blocking the PD-1 receptor, which binds ligand PD-L1 on tumors, blunting the immune response. Recent research on cemiplimab includes a study that showed a 47-percent response rate among 108 patients with advanced cutaneous squamous cell carcinoma.
Other topics discussed by Drs. Rosen and Bhatia include:
- Treatment success in a study comparing tretinoin in a new lotion for acne versus vehicle over 12 weeks.
- Relatively new FDA-approved antibiotics including delafloxacin, ozenoxacin, omadacycline, and sarecycline.
- New antiviral tecovirimat for smallpox.
- Mogamulizumab for cutaneous T-cell lymphoma.
- Lanadelumab for hereditary angioedema.
- Tapinarof for psoriasis.
- 1.5% minocycline foam for the treatment of moderate-to-severe papulopustular rosacea.
- Topical nitric oxide-releasing drug for extragenital wart treatment and molluscum.
- Hypochlorous acid for acne.
- JAK Inhibitors for atopic dermatitis, vitiligo, and alopecia.