Botulinum Toxin: Science and Evidence Data, Dose, Duration, Dogma
Joel L. Cohen, MD
In this presentation, Dr. Cohen discusses the neuromodulators that we commonly use and how to best apply the science and evidence into clinical practice. Dr. Cohen spends about 40 percent of time in practice doing Mohs surgery and the other 60 percent is dedicated to aesthetics.
It’s important to remember that we typically don’t focus in one area but rather treat assess patient’s full face and multiple regions, and we tend to use a combination of therapies/treatments (neuromodulators, fillers, lasers, and other energy-based devices such as radiofrequency and ultrasound). Clinical research is important to us, as clinicians, for a number a reasons — not only does it allow us to see what products are coming up on the horizon, but it affords us the opportunity to experience these therapies first-hand in clinical practice.
Currently, we have three neuromodulators approved for aesthetic use; onabotulinumtoxinA ((Botox), abobotulinumtoxinA (Dysport) and incobotulinumtoxinA (Xeomin). The important concept here is that these products are probably more similar than they are different.
BTX-A On-label Aesthetic Uses
Of note, when you go to other countries, especially in Europe, you will see a different spectrum and wider-spectrum of approved aesthetic indications for these products.
We know, from clinical data, that botulinumtoxin can make patients feel better about themselves and can help them outwardly convey their inner emotions more appropriately. Many patients want to delay the outward appearance of aging as well as simply “look their best”. (Finn, Cox, Earl. Social Implications of Hyperfunctional Facial Lines. Dermatol Surg 2003;29:450-455.)
A double-blind, randomized, placebo-controlled health outcomes survey conducted by Dayan and colleagues analyzed the effect of botulinumtoxin type A injections on quality of life and self-esteem. The researchers found that the injections result in improvements in quality of life (QOL) and self-esteem. Additionally, botulinumtoxin-naïve patients demonstrated greater improvements in QOL and self-esteem than participants previously exposed to botulinumtoxin. Moreover, botulinumtoxin-familiar patients demonstrated sustained improvement in QOL and self-esteem as compared to botulinumtoxin-naïve patients, even when injected with placebo.
All of the approved botulinumtoxin type A products have a 150 kD botulinumtoxinA core neurotoxin protein. Incobotulinumtoxin (Xeomin) lacks the accessory proteins that are naturally produced by clostridial bacteria, which Botox and Dysport maintain.
When you look at the clinical studies, remember that there are different comparisons and different endpoints. Non-inferiority studies tend to compare two products at very specific time points. One noninferiority study compared incobotulinumtoxinA to onobotulinumtoxinA at four weeks and 12 weeks, as assessed by investigators, a panel of independent raters, and patients. This study demonstrated that incobotulinumtoxinA is equally as effective as onabotulinumtoxinA in the treatment of glabellar frown lines and both products were well tolerated — at the specific timepoints studied. There are; however, issues with non-inferiority studies. Because there are two time-points, there may be a missed evaluation of the duration of efficacy and a “waning effect.”
If you look at different demographics of your study cohort in some clinical trials, you’ll see that in some studies it’s really reflective of what we do in clinical practice; however, in other studies, it may not be – such as a disproportionate number of patients being quite young. These are issues that need to be considered when we’re looking at overall responses and comparisons among different products.
We also need to think about “low-powered” comparisons. In a comparison of two botulinum toxin type A preparations for the treatment of crow’s feet (Prager, et al), there were only 21 patients in this study. This may not be enough to tease out any major differences between the products in terms of the number of patients studied.
So, in short, there are 3 botulinum toxin products available in the US. It’s very difficult to make direct comparisons. These are different products and we need to consider the fact that the dosing can be different and the studies can be designed differently in terms of non-inferiority, demographics and power as well as an evolution of different study endpoints (2-grade improvement versus 1-grade).
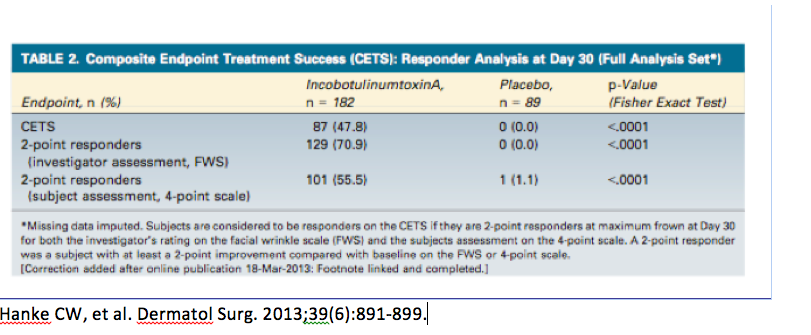
We are beginning to see a shift towards more stringent primary endpoints. A randomized, double-blind, placebo-controlled phase III trial, conducted by Hanke and colleagues, investigated the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using Composite Endpoint Treatment Success (CETS), i.e., looking at a two-grade improvement. If you look at how often physicians saw a two-grade improvement versus patients, you will see about 48 percent.
Remember that it’s difficult to compare data as previous studies often used a one-grade improvement; therefore, demonstrating a larger responder rate and a longer duration of treatment.
The pivotal trial with Botox for the lateral canthal lines required at least a two grade improvement from baseline on both the investigator’s and subject’s assessment of CFL severity at maximum smile using the 4 facial wrinkle scale. At day 30, we see a responder rate of 25.7%. Dr Cohen states that we know we have seen higher responder rates than this; however, because there has been a chance in the way in which the FDA wants this to be assessed, i.e., patient and physician composite scores, the numbers are lower.
What about conversion ratios?
Again, remember that these are different products and we need to look at them as such. Various studies have looked at the concept conversion ratios, but that may be difficult to compute in clinical practice. Regulatory agencies worldwide have recognized that these products are not interchangeable.
Some Other Things to Consider…
When we look at patients, specifically speaking about the lateral canthus, not everyone needs the exact injections that were used in clinical trials. There have been studies, dating back to 2003, looking at different patterns of injections and the way that different people look. It’s important to identify these features in your patients and individualize therapy. When Dr Cohen looks at a patient and he/she is superior dominant, he may give more of the neuromodulator up high, and a little bit less in the middle or below. Keep in mind that on that same horizontal is the bunny line and the contraction of the nasalis. Also along this horizontal is the pharmacologic brow lift.
If we understand the anatomy that the orbicularis oculi pushes our brows down and the frontalis lifts them up, then if we inject the one site where you’re getting the maximum pull down below the lateral, you may see better improvement in the lateral brow lift we can sometimes see when we treat the lateral orbicularis oculi.
Drs Cohen and Dayan conducted an open-label, randomized, dose-comparison study of botulinum Toxin Type A in the treatment of dermatochalasis. They found that a single-site injection of botulinum toxin type A in the lateral infrabrow can offer effective treatment for mild to moderate upper eyelid dermatochalasis—with perhaps a couple of millimeter lateral brow lift at best.
We know that when we look at patient’s brow positioning, there are some that should not be injected with a neuromodulator in the off-label area of the forehead. There was an important article that was published in JAMA Dermatology last year that looked at not only a grading scale for dermatochalasis, but it also discussed the factors associated with dermatochalasis. (Jacobs L, et al, 2014) From a grading scale, there are four categories for sagging eyelids (mild, moderate, severe, very severe). Specific risk factors associated with sagging eyelids include, age, male sex, lighter skin color, smoking status and higher BMI. In many cases, people “blame” genetics for sagging eyelids; however, we can see that there are things that we can do to intervene to help reduce the risk.
Combination Delivery
After the approval of Radiesse with in-office adding of anesthesia in the same syringe, some physicians began to use the same adaptor to combine fillers and neuromodulators. Dr Cohen doesn’t feel that this is a good idea; in fact, he believes that it probably does not save time, leads to an unstandardized mixture, and likely leads to less precise injections – as there are simply some areas where you don’t want toxin but you do want filler. So combination in the same syringe of toxin and filler in Cohen’s view is not a good idea, but combination of toxin and filler in their respecitive different syringes is still a good concept.
When it comes to the lower face and the area around the mouth, a study conducted by Carruthers et al indicated that both physicians and patients saw a greater improvement when the patient was injected with both the hyaluronic acid dermal filler and a neuromodulator. (Carruthers A, et al. Dermatol Surg. 2010;36:Suppl4:2121-2134.
And other studies have shown synergy with the combination treatment of different regions with toxin and filler. A 2003 study of Botox plus Restylane demonstrated that if you inject patients with Botox and then have them come back for Restylane, it has a longer tissue residence time in terms of filler and correction than if you injected Restylane alone (18 weeks versus 32 weeks.)
Dosing
Many of us have seen an overall change in practice patterns when it comes to dosing. In some areas, like especially the forehead, we have shifted from higher doses of toxin to lower doses in order to achieve a more natural, relaxed look for our patients. In the forehead, for example, Dr Cohen and other colleagues who participated in the more recent PRS journal consensus, now inject about half of the dose they previously used in the forehead — thus creating often a more natural look, simply softening the musculature, and maintaining brow shape and positioning better. There’s a lot to consider with regards to neuromodulators. We have seen where NOT to inject, but it’s important to maintain brow positioning and shape as well as symmetry.
There are very specific grading scales that can be useful in clinical practice and Dr Cohen recommends incorporating them into your regimen. This is helpful when discussing the goals of therapy with patients—for instance, for the forehead “softening of the musculature”.