Keith T. Flaherty, MD
At Maui Derm 2015, Dr Flaherty, a medical oncologist at Massachusetts General Hospital, reviewed updates in melanoma therapy for advanced disease and how these drugs have impacted clinical care. As we try to build on targeted therapy based on our current knowledge of pathways, we ask ourselves what will be the role of combinations and/or precision medicine-type immune therapy matching strategies. Of note, the therapies discussed are investigated in the metastatic setting first, and as dermatologists, we are considering their use in the adjuvant setting.
Ipilimumab
Ipilimumab was the first entry into the field in terms of a positive phase III trial. Ipilimimab at 3mg/kg, with or without gp100, was given every three weeks for four treatments. Based on the data, there’s no question that ipilimumab outperformed the gp100 vaccine alone. This outcome, at the level of overall survival, demonstrated the efficacy needed to warrant the approval of ipilimumab.
Click to Enlarge
Ipilimumab was already a ten-year old therapy for which metastatic melonama patients were taking the drug for a few months course of treatment and who are still alive and well. Dr Flaherty states that we knew, when these results emerged, that there may be a truly, durable, lasting effect.
There is a cost to this type of treatment in terms of adverse events (AEs). All of the toxicities associated with this drug come from autoimmune attack(s) affecting the skin, large intestine, endocrine glands, or hepatic autoimmune injury. Why these tissues and not others? There are several theories, but not a firm understanding. If you focus on the ipilimumab monotherapy group, you can look at those who had severe autoimmune toxicity, i.e., you have to stop giving the drug and/or give high-dose corticosteroids to stop the autoimmune reaction. This occurs about 10 percent of the time. Everything short of this autoimmune toxicity appears, in short, in mild or moderate form and basically goes away without any immunologic intervention.
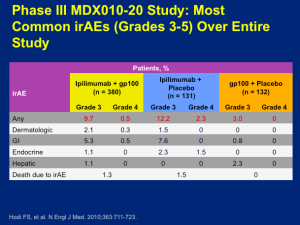
Click to Enlarge
The idea is that we think we’re unmasking normal tissue tolerance, but in a largely reversible way. About two thirds of patients who receive ipilimumab have some form of autoimmune toxicity, only about 10 percent have enough of it that it creates a truly life-threatening scenario for which we need to intervene. Not all of the toxicities manifest at the same time. Dr Flaherty feels that in major melanoma programs, we have become quite comfortable with regards to counseling patients as far as what to look out for and when to call with suspected reaction(s). Dr Flaherty believes that ipilimumab is a therapy that can absolutely be safely administered.
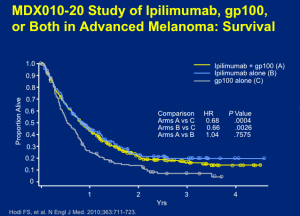
The other phase III trial that corroborated the effects of ipilimumab looked at patients taking ipilimumab (10mg/kg) plus decarbazine (850 mg/m2) or dacarbazine (850 mg/m2) plus placebo at weeks one, four, seven, and ten, followed by dacarbazine alone every three weeks through week 22. This trial was similarly positive with the addition of ipilimumab. The difference, as shown below, was really not very different from that of the ipilimumab versus vaccine trial.
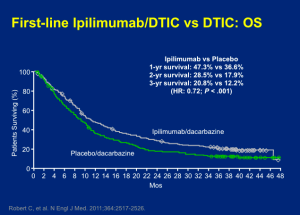
Click to Enlarge
The toxicities of the two-drug approach were modestly worse. When the FDA looked across the two data sets, they didn’t feel that the addition of chemotherapy was synergistic; therefore, they went with the previous study and approved ipilimumab as a monotherapy for metastatic melanoma.
In clinical practice, you can look at the pooled data set (below) and feel comfortable counseling patients that they have about a 20 percent chance of walking away with the diagnosis of metastatic melanoma by receiving this treatment (four doses over three months) with time-limited risk of toxicity.
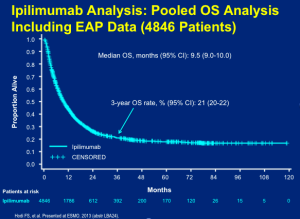
Click to Enlarge
We have learned that if you “cherry-pick” patients and only treat those with lower disease burden, the rate may be as high as 30 percent.
The PD-1 Pathway
What is the role of the PD-1 pathway in suppressing anti-tumor immunity? How might we think about deploying antibodies that try to intercept this negative immune regulator? We can target on either the PD-1 side or the PD-L1 side [with antibodies]. Scientifically, we would anticipate that this would alleviate the negative influence that the expression of this immune marker on tumor cells can produce in terms of silencing T-cells directly. There was a belief that PD-L1 target is actually safer than PD-1 targeting because you don’t disrupt PD-L2/PD-L1 interactions that a PD-1-blocking antibody would and there is some clinical evidence that this may be true.
Pembrolizumab
Pembrolizumab was FDA-approved in the late summer of 2014. In the pivotal, randomized study, patients who had received ipilimumab and/or a BRAF inhibitor, if they were BRAF mutant, were randomized to receive pembrolizumab 2mg/kg IV every three weeks (ongoing), pembrolizumab 10mg/kg IV every three weeks (ongoing) or chemotherapy. This was a trial that was aiming to show that patients who had exhausted the available therapies could benefit from treatment with pembrolizumab. This data set addresses the unmet need of patients who don’t get a benefit from ipilimumab.
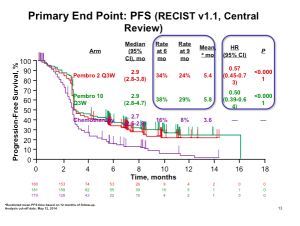
Click to Enlarge
The data (above) is impressive when looking at pembrolizumab compared to chemotherapy at the level of progression-free survival. This is clearly a positive study and very important data for patients who had exhausted all of the other treatment options. It is true; however, that this therapy does not work for all patients and about half of patients will still experience disease progression. The number of patients who have objective responses, again these are patients who have not exhausted all other options, appears to be about 20-25 percent.
Two different doses were investigated in this study (2 mg/kg Q3W and 10mg/kg Q3W). Because there was no difference in efficacy, the lower dose, which is modestly safer, was the regimen that was approved. The toxicity is identical in type with ipilimumab. This type of immune therapy produces the same autoimmune reactions in terms of the affected organs and tissues. The issue is that the severity is clearly lower than that of iplimumab.
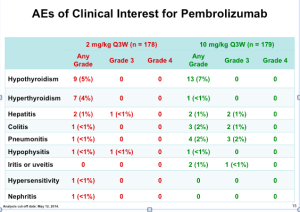
Click to Enlarge
If you look at the Grade 3 and Grade 4 AEs (above), notice how there are only a few autoimmune toxicities in the approved pembrolizumab group (2mg/kg).
For our patients, this is a drug that can be effective and a bit less dangerous in terms of autoimmune toxicities.
Nivolumab
Nivolumab, another PD-1 antibody that was approved in December 2014, was studied in patients who had not received prior therapy. Patients were randomized to nivolumab (3 mg/kg IV every two weeks) plus placebo or placebo plus dacarbazine (1000 mg/m2 IV every three weeks). The thought here was that if you were going to conduct a clinical trial that was chemotherapy controlled, you couldn’t include BRAF-mutant patients because you wouldn’t offer them decarbazine as a front-line “cytotoxic” therapy. Patients in this study were stratified based on PD-L1 status.
This drug has a huge impact in terms of overall survival.
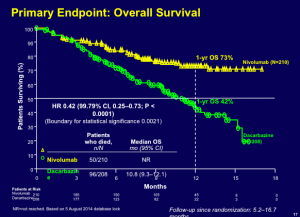
Click to Enlarge
The data demonstrate a one-year overall survival rate of 73 percent. Many practitioners look at the evidence and would like to get PD-1 antibodies in the front-line. Currently, these drugs are approved for second- or third-line.
Progression-free survival clearly improved as well with nivolumab. These are immune therapies, but they have a clear impact on tumor burden as well. About sixty percent of patients have some demonstrable tumor effect.
When you see a response, the likelihood that the response is going to be durable through six and twelve months of follow-up is very likely.
PD-L1 status is an interesting biomarker to which we should pay attention. The overall survival outcome for patients whose tumors express PD-L1 on their surface does better than the control group and the overall population. (see below)
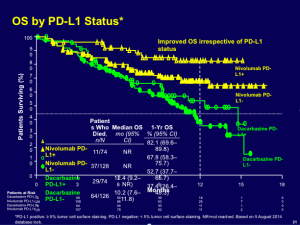
Click to Enlarge
It is fairly clear that we can do some enrichment by using PD-L1 on the tumor as a biomarker.
Combination Therapy
In the medical oncology metastatic melanoma field, there is a lot of research around the concept of combining PD-1 and CTLA-4-blocking antibodies. Tumor responses in patients receiving nivolumab plus ipilimumab demonstrated that approximately 90 percent of patients responded after a follow-up of about 13 months.
Cli
There is still a group of patients who remain refractory, even to two-drug therapy. This phase I data ultimately launched a large phase III trial and we expect to see the data in June of this year (2015). The combination regimen cannot be given at the full dose of each drug; at least one of the therapies has to be attenuated. The data in the phase III trial is not quite as robust; however, we look forward to hearing the results in June.
When you look at the best available data with PD-1 monotherapy (pembrolizumab), this is actually not bad (below). Dr Flaherty feels that it is still unclear as to whether combination therapy is lifting us to a new plateau. We need more evidence to support that. Regarding safety, in patients who were taking combination therapy at the doses that were taken into the phase III trial, there was a sixty percent rate of severe (Grades 3 and 4) autoimmune toxicity. The results from the phase III data will help us to determine whether or not this combination therapy can be clinically useful.
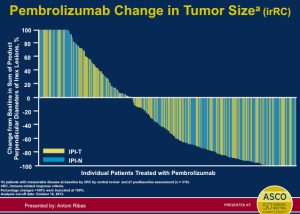
Click to Enlarge
Adjuvant Therapy
There is some preliminary evidence on adjuvant therapy as we only have an interim result from a trial looking at ipilimumab monotherapy versus placebo in 951 patients with high-risk, stage III, completely resected melanoma. The primary endpoint was recurrence-free survival. If you focus on hazard ratio, there is about a 25 percent improvement. The question is whether or not this drug is really doing something more effective in the adjuvant setting than it would otherwise do in the overt metastatic setting. This is not clear—we need to see more data, more follow-up, and more overall survival evidence to know if this is a therapy that should be moved to the high-risk, resected setting. This therapy can have significant toxicity consequences, so we do have reason to be concerned.
Toxicities are substantial in the adjuvant setting. Notice the rate of Grade 3 and 4 toxicities. (below) This is the same drug that has a ten to twelve percent toxicity rate in the overt metastatic setting, but more in the adjuvant setting—partly because of the higher dose.
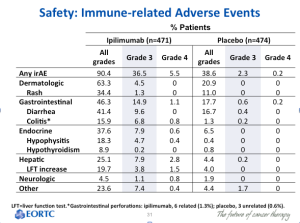
Click to Enlarge
There were treatment-related deaths in this trial. This is not something that we can take lightly.
Vaccines
From 2010 to 2015, in the metastatic melanoma field, we have run about twelve phase III trials and they have all been positive, except for the most recent of the vaccine trials, a MAGE-3 peptide. Prior to this, we have had three peptide vaccine trials suggesting a detriment in overall survival. We still have not found a way to deploy these therapies. Dr Flaherty states that many of us are hopeful that either by using some biomarker selection strategy or, more likely, using these agents with immune checkpoint antibodies, we may be able to find utility in steering immune responses towards specific epitopes. Currently, there are no ongoing phase III trials.
Conclusions
Ipilimumab was the first-in-class immune checkpoint inhibitor and has demonstrated survival advantage. Long-term follow-up clearly supports survival impact with only three months of therapy. PD-1/PD-L1 is considered “best-in-class” based on higher response rates and better disease control; however, the percentage rate of long-term survivors is unknown. The combination of ipilimumab/nivolumab suggests synergistic toxicity and the question remains regarding synergistic efficacy. The adjuvant role of therapy is still not defined as we only have interim data for ipilimumab at the higher dose.