New Developments in the Treatment of Moderate-to-severe Psoriasis: Biologics
Based on the Maui Derm 2018 presentation by Craig L. Leonardi, MD, Associate Clinical Professor of Dermatology, St. Louis University Medical School, St. Louis, Missouri
Article by Jo Ann LeQuang and the Journal of Clinical and Aesthetic Dermatology
Cytokines, those small proteins that regulate specific activities of a wide range of immunocompetent cells, are only beginning to be elucidated. Their main known function can be described, perhaps simplistically, as regulating the communication among cells within and outside of the immune system. It is quite possible that not all cytokines have been discovered, but recent advances have identified several and their in-vivo functions. The main groups of cytokines are the pro-inflammatory/anti-inflammatory cytokines (described here), cytokines related to neutrophil and eosinophil recruitment and activation, Th cell cytokines, T-regulatory (Treg) cell cytokines, and cytokines involved in the recruitment and growth of T-cells. Signaling pathways within the nucleus and cytoplasm of the cell connect cytokine receptors; these pathways can activate cytokines (along with other factors) during or after transcription.98,99
Cytokines appear to be the main mediators of the cutaneous inflammation associated with psoriasis.100 The cytokine network is extremely complex: cytokines can change the expression of receptors which, in turn, can change how responsive a source and/or target cell are to the cytokine. In treating psoriasis, targeting specific cytokines for inhibition has reduced chronic inflammation and led to major advances in controlling the disease. The main families of cytokines associated with psoriasis are TNF alpha, IL, and TGF beta.101 Humanized monoclonal antibodies (the -mab suffix drugs) target specific cytokines, and some have shown promise in the treatment of psoriasis. The cytokines associated with psoriasis appear in Figure 1, many of which have become important new targets for drug development.102
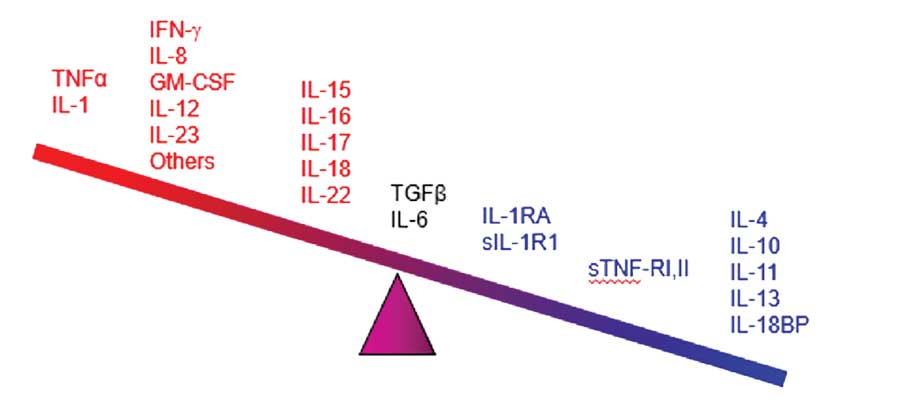

Figure 1. The cytokines associated with psoriasis ranging from the most pro-inflammatory (far left) the most anti-inflammatory (far right)
Certolizumab-pegol. Certolizumab-pegol (CZP) is a monoclonal antibody that targets TNF alpha. It is a PEGylated antigen-binding fragment (Fab) of a humanized TNF-inhibitor monoclonal antibody. CZP has been approved for use by the FDA for the indications of Crohn’s diseases (2008), rheumatoid arthritis (2009), active psoriatic arthritis (2013), ankylosing spondylitis (2013), and most recently for the treatment of psoriasis.103 The CIMPASI-1 (n=234) and CIMPASI-2 (n=227) placebo-controlled trials104 evaluated SQ CZP in patients with moderate-to-severe plaque psoriasis, scored based on the PASI and PGA scores, and found significant improvements in both active groups versus placebo at 16 weeks. In CIMPASI-2, patients were randomized to one of three treatment arms: 400mg SQ CZP every two weeks (n=87), 400mg SQ CZP at Weeks 0, 2, and 4 followed by 200mg every two weeks thereafter for 16 weeks (n=91), and placebo every two weeks (n=49). The primary endpoint of the study was meeting PASI75 at 16 weeks, which was achieved by 82.6 percent of the first group (400mg every 2 weeks), 81.4 percent of the second group (400/200mg), and 11.6 percent of placebo patients. The PGA endpoint called for improvement of at least two points by 16 weeks; 71.6 percent, 66.8 percent, and 2.0 percent met this endpoint in the first, second, and placebo groups, respectively. Thus, CZP demonstrated significant improvements from baseline at 16 weeks compared with placebo for both PASI75 and PGA endpoints. Adverse events were similar to those reported when using CZP for other approved indications.104,105
Guselkumab. Guselkumab is an IL23-P19 inhibitor and was evaluated in a 48-week, Phase III, double-blind, placebo-controlled VOYAGE 1 trial.106 Patients were randomized into one of three groups: 1) patients (n=329) received guselkumab 100mg (Weeks 0 and 4 and then every 8 weeks), 2) patients (n=174) started with placebo at Weeks 0, 4, and 12 and then rotated to guselkumab at Weeks 16 and 20 and every eight weeks thereafter, and 3) patients (n=334) received adalimumab 80mg at Week 0, 40mg at Week 1, then 40mg every two weeks through Week 47. Endpoints were the PASI score, the patient-reported outcomes on the Dermatology Life Quality Index, and the Psoriasis Symptoms and Signs Diary. Guselkumab demonstrated significant superiority (p<0.001) to placebo at 16 weeks (85.1% vs. 6.9% on Investigator Global Assessment Score [IGA]) and 73.3 percent versus 2.9 percent showed a 90-percent or greater improvement in PASI score over baseline. When compared to adalimumab, guselkumab was significantly superior (p<0.001) in IGA and PASI90 at Weeks 16, 24, and 48.106 During the placebo-controlled phase of the study, at least one adverse event was reported by 49.4 percent, 51.7 percent, and 51.1 percent of the placebo, guselkumab, and adalimumab groups, respectively; over the entire course of the study, 73.9 percent of the guselkumab group and 74.5 percent of adalimumab group reported at least one adverse event. In the group that transitioned from placebo to guselkumab, 64.8 percent of patients reported at least one adverse event during Weeks 16 to 48. Serious adverse events were reported by less than five percent of all patients; 4.9 percent of guselkumab and 4.5 percent of adalimumab patients reported at least one serious adverse event over the course of the study.106 In a Phase II study of guselkumab, ACR 20/50/70 scores showed the significant superiority of guselkumab over placebo. 107
Tildrakizumab. Like guselkumab, tildrakizumab targets IL-23 p19 and was shown in two Phase III clinical trials to be more effective than placebo and etanercept for treating moderate-to-severe plaque psoriasis.108 In these international multicenter, three-part, parallel-group, randomized, double-blind studies, patients were randomized to tildrakizumab 200mg, tildrakizumab 100mg, or placebo (reSURFACE-1 N=1,772) or to tildrakizumab 200mg, tildrakizumab 100mg, placebo, or etanercept 50mg (reSURFACE-2 , N=1,090). In the reSURFACE-1 study, at Week 12, 192 patients (62%) in the 200mg group and 197 patients (64%) in the 100mg group achieved PASI75, compared with nine patients (6%) in the placebo group. In the reSURFACE-2 study, at Week 12, 206 patients (66%) in the 200mg group, and 188 patients (61%) in the 100mg group achieved PASI75, compared with nine patients (6%) in the placebo group and 151 patients (48%) in the etanercept group. Serious adverse events were similar and low in all groups in both of these trials. One patient died in reSURFACE-2 (tildrakizumab 100mg group) but the cause of death could not be determined. The FDA approved tildrakizumab for market release in March 2018 for the treatment of plaque psoriasis.
Risankizumab. Risankizumab targets IL-23 p19, and the IMMhance trial evaluated its use against placebo over 16 weeks, but results are not yet published. From data on file at AbbVie, preliminary results found that 73.2 percent of risankizumab patients achieved PASI90 and 47.2 percent PASI100 at 16 weeks compared with 2.0 percent and 1.0 percent for placebo, respectively. In a recent study of 166 patients with moderate-to-severe plaque psoriasis,92 patients were randomized to receive subcutaneous risankizumab (one 18mg dose at Week 0 or 90 or 180mg doses at Weeks 0, 4, and 16, or ustekinumab (45mg or 90mg based on body weight at Weeks 0, 4, and 16). At Week 12, of the patients who had a 90-percent or greater reduction in their PASI score, 77 percent were in the risankizumab group (pooled 90mg and 180mg group results) versus 40 percent in the ustekinumab group (p<0.001). Results demonstrated durability up to 20 weeks for risankizumab. A serious adverse event was reported by 12 percent of patients in the risankizumab 18mg group, 15 percent in the risankizumab 90mg group, and eight percent ustekinumab, including one MACE and two BCCs. No serious adverse events were reported in the risankizumab 180mg group.92
Mirikizumab. A relatively new monoclonal antibody to be evaluated for the treatment of moderate-to-severe plaque psoriasis is mirikizumab, which targets IL-23 p19. A Phase II randomized, multicenter, double-blind, placebo-controlled trial is being conducted using different doses of mirikizumab and evaluating them for safety and efficacy is ongoing [ClinicalTrials.gov Identifier: NCT02899988]. Results of this study were presented at the 2018 American Academy of Dermatology Annual Meeting [Abstract # 6131] by Rich et al. In this study, 205 patients were randomized to mirikizumab 300mg every eight weeks (Q8W), 100mg Q8W, 30mg Q8W, or placebo Q8W over the course of 16 weeks. The mirikizumab 300mg and 100mg groups were significantly more likely to achieve PASI90 at 16 weeks compared to placebo (67% and 59%, respectively vs. 0 in placebo, p<0.001). Twenty-nine percent of the mirikizumab 30mg group achieved PASI90 at 16 weeks, but this was not significant compared to placebo. PASI100 scores were achieved by significantly more patients in the mirikizumab 300mg and 100mg groups than the placebo group at 16 weeks (31%, p=0.007 for both); 16 percent of the patients in the mirikizumab 30mg group achieved PASI100 at 16 weeks, which was significant versus placebo (0%, p=0.039). Pooling safety results from all mirikizumab groups, at least one TEAE was reported by 48.4 percent of mirikizumab patients compared with 48.1 percent of the placebo patients, and 1.3 percent of the mirikizumab patients and 1.9 percent of the placebo patients reported at least one serious adverse event.
Secukinumab. The IL-17 family of cytokines includes IL-17A, IL-17A/F, IL-17F, IL-17E, and IL-17C, and secukinumab targets IL-17A. There are long-term data for secukinumab showing that five-year PASI score improvements are durable over time.109 At Year 1, 168 patients entered the extension study, and at the end of Year 5, 126 patients completed 300mg (every 4 weeks) treatment. PASI 75/90/100 responses at Year 1 (88.9%, 68.5% and 43.8%, respectively) were sustained to Year 5 (88.5%, 66.4% and 41%, respectively). Results from Future 5, a placebo-controlled study, evaluated 300mg and 150mg of secukinumab and 150mg secukinumab with no loading dose compared with placebo,89 and in this study, significantly more patients achieved PASI75 and PASI90 with secukinumab compared to placebo.
Ixekizumab. Ixekizumab targets IL-17A, and the UNCOVER-3 trial provides long-term data with durable efficacy up through 108 weeks. In this randomized, controlled, Phase III study, 1,346 patients with moderate-to-severe plaque psoriasis were randomized to one of four treatment arms.110 Patients received ixekizumab 80mg every two or four weeks, etanercept 50mg twice a week, or placebo. Patients entered the long-term extension phase at Week 12 when all patients were switched to ixekizumab every four weeks.
The results of a head-to-head comparison of ixekizumab compared to ustekinumab111 reported that 52.2 percent more patients in the ixekizumab group achieved PASI100 scores at 52 weeks compared to those in the ustekinumab group (35.5%).
Brodalumab. In contrast to ixekizumab, which binds to IL-17, brodalumab binds to the IL-17 receptor and, in that way, prevents IL-17 from activating that receptor. In two Phase II studies comparing brodalumab to ustekinumab in patients with moderate-to-severe plaque psoriasis, brodalumab was associated with significant clinical improvements compared to ustekinumab.112 At 12 weeks, brodalumab 210mg resulted in 44 percent of patients achieving PASI100 scores compared with 22 percent of the patients taking ustekinumab in AMAGINE-2; these results were similar to AMAGINE-3, where 37 percent of patients in the brodalumab group achieved PASI100 scores compared to 19 percent of patients in the ustekinumab group (p<0.001, both). Rates of neutropenia were higher with brodalumab and ustekinumab versus placebo. Safety issues with brodalumab surfaced around this time when a numeric imbalance was noted in terms of cases of depression, suicidal ideation, and suicidal behavior. During the Phase III brodalumab trials, the manufacturer introduced psychological surveys to help detect patients at risk but the intervention was not successful. All trials were halted in the second quarter of 2015, but brodalumab was eventually approved by the FDA in 2017 with a black box warning.113 It should be noted in this context that, overall, patients with psoriasis appear to have higher rates of suicidal ideation than the general population. A 1993 survey found that 5.5 percent of patients with psoriasis had active suicidal ideation at the time of the study, and the more severe the patient considered his or her psoriasis, the more likely the patient was to have suicidal thoughts.114
Bimekizumab. Bimekizumab has a novel dual mechanism of action in that it neutralizes both IL-17A and IL-17F, two key pro-inflammatory cytokines.115 Based on clinical data on file with UCB, in the Phase II BE ABLE clinical study, 79 percent and 60 percent of bimekizumab patients achieved PASI90 and PASI100 scores, respectively, in 12 weeks. The safety and efficacy of bimekizumab was demonstrated in a first-in-human randomized clinical trial assessing patients with mild plaque psoriasis.116 Based on early results, this new agent appears to hold promise for the treatment of psoriasis.
The paradigm shift and future directions. The classic treatment of psoriasis had always been a stepwise progression from over-the-counter products to prescription topicals to phototherapy and then, if these treatments were no longer effective, finally to systemic therapy. In all cases, patients were expected to receive treatment in an orderly sequence, step by step, and not skip any steps. Aggressive therapy was reserved only for those patients who failed more conservative approaches. Today, the evolution of new drugs for psoriasis and the increased understanding of its pathogenesis have resulted in our discarding the classic paradigm in favor of a new approach. If a patient with psoriasis is an appropriate candidate for topical treatment, that should be the first-line approach; if that fails or the patient is not a good candidate for topical therapy, then there are three main types of therapy: biologic agents, traditional systemic agents (such as methotrexate), and phototherapy. The choice of therapy must be based on the individual characteristics of the patient. Within each group there are further therapeutic choices, again based on the needs of the individual patient. There is no longer any need for a patient to have to progress through and fail multiple treatment steps before receiving biologic therapy.
References
98. Nickoloff BJ. Cracking the cytokine code in psoriasis. Nature Med. 2007;13(3):242–244.
99. Guttman-Yassky E, Krueger JG. Psoriasis: evolution of pathogenic concepts and new therapies through phases of translational research. Br J Dermatol. 2007;157(6):1103–1115.
100. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33(1-2):45–56.
101. Nickoloff BJ, Xin H, Nestle FO, Qin JZ. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25(6):568–573
102. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873.
103. Drugs.com site. Development history and FDA approval process for Cimzia. Cimzia approval history 2018; https://www.drugs.com/history/cimzia.html. Accessed May 25, 2018.
104. McKnight W. CIMPASI-1 and -2 show certolizumab pegol benefits patients with severe plaque arthritis. Conference Coverage 2017; https://www.mdedge.com/edermatologynews/article/132706/psoriasis/cimpasi-1-and-2-show-certolizumab-pegol-benefits-patients. Accessed May 25, 2018.
105. Global Newswire site. CIMZIA (certolizumab pegol) Phase 3 trial meets co-primary endpoints in patients with moderate-to-severe chronic plaque psoriasis. Dermira press release. 8 Dec 2016. https://globenewswire.com/news-release/2016/12/08/895986/0/en/Second-CIMZIA-certolizumab-pegol-Phase-3-Trial-Meets-Co-primary-Efficacy-Endpoints-in-Patients-with-Moderate-to-Severe-Chronic-Plaque-Psoriasis.html. 27 Sep 2018.
106. Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
107. A Deodhar, A Gottlieb, W-H Boehncke, et al. Efficacy and safety results of guselkumab in patients with active psoriatic arthritis over 56 weeks from a phase 2a, randomised, double-blind, placebo-controlled study. Abstract # OP0308. Presented at Annual European Congress of Rheumatology. Madrid, Spain: 14–16 Jun 2018. Ann Rheum Dis. 2018;77 (Suppl 2). https://ard.bmj.com/content/77/Suppl_2/201.1. Accessed 27 Sep 2018.
108. Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
109. Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-tosevere psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–1514.
110. Blauvelt A, Gooderham M, Iversen L, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3). J Am Acad Dermatol. 2017;77(5):855–862.
111. Paul C, Griffiths CEM, van de Kerkhof PCM, et al. AIxekizumab provides superior efficacy compared to ustekinumab over 52-weeks of treatment: results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2018 Jun 30. pii: S0190-9622(18)32195-9. doi: 10.1016/j.jaad.2018.06.039. [Epub ahead of print].
112. Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New Engl J Med. 2015;373(14):
1318–1328.
113. United States Food and Drug Administration site. FDA Briefing Document. Background Package for BLA 761032 Siliq (brodalumab) injection, 210 mg/1.5 ml. Dermatologic and Ophthalmic Drugs Advisory Committee. meeting. July 19, 2016. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/ drugs/dermatologicandophthalmic drugsadvisorycommittee/ucm511357.pdf. Accessed 27 Sep 2018.
114. Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32(3):188–190.
115. Lonnberg AS, Zachariae C, Skov L. Targeting of interleukin-17 in the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2014;7:251-259.
116. Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991-1001.