Alain H. Rook, MD
At MauiDerm 2014, Dr Rook provided the audience with an update on CTCL, a challenging disease in the field of dermatology. Dr Rook, one of the world’s leading authorities on CTCL, is a professor at the Perelman School of Medicine at the University of Pennsylvania.
There is one scenario that unfortunately occurs all too often with patients who present with erythroderma—you must be absolutely sure of the diagnosis before you administer therapies (anti-TNF agents, ustekinumab, cyclosporine, etc) that will blunt the immune response. This is extremely important because you do not want to administer these agents in patients with CTCL as they can lead to rapid disease progression.
Diagnosis of Erythroderma
In an erythrodermic patient, dermatologists should be aware that a single biopsy is inadequate in up to fifty percent of cases due to the absence of critical diagnostic features.
What do we look for in a biopsy?
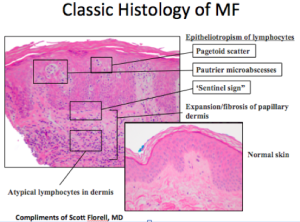
When looking at a biopsy, we look for well-known epidermotropic morphology. We also look for clusters of cells. Another critical finding is the lining up of some large lymphocytes at the dermal/epidermal junction. The malignant T-cells are being recruited towards the epidermis by chemokines, with the “sentinel sign” a characteristic that is more often found in erythroderma than is epidermotropism. We also need to look for large atypical cells in the dermis occasionally in association with eosiniphils. Eosiniphilia, together with erythroderma, should not equal pityriasis rubra pilaris or psoriasis, but is more characteristic of erythroderma associated with either atopic dermatitis or cutaneous T-cell lymphoma (CTCL).
In up to fifty percent of cases, there is a high frequency of non-diagnostic histology in Sezary syndrome, the leukemic variant of CTCL. Published studies have observed chronic dermatitis in 33 percent of biopsies from Sezary syndrome and no evidence of CTCL in 25 percent of biopsies from these patients. “Non-specific cutaneous histopathologic findings are common in Sezary syndrome.” (Trotter MJ. J Cut Pathol. 1997)
In addition, we like to study both the skin and the blood and determine if there is a dominant T-cell clone. In particular, if you see matching clones in the skin and blood, that’s usually typical of CTCL, but not always. Autoimmunity can be associated with dominant clones; however, you typically do not see the identical clone in the skin and blood in a patient with autoimmune disease. This is much more characteristic of CTCL or a T-cell lymphoma.
Flow cytometry is absolutely critical to perform on an erythrodermic patient in whom you would like to rule out CTCL. Prior to about ten years ago, the gold standard was looking for the loss of CD7 on CD4 T-cells that is known to occur in about 50 percent of leukemic patients. Now, we typically look for loss of CD26, occurring in greater than 90 percent of leukemic patients in our experience. This is important because in more than 80 percent of active erythrodermic CTCL cases, you will see active peripheral blood involvement or leukemia. This is a critical test to perform in a highly skilled laboratory. Many of the public laboratories that are not associated with academic programs do not monitor for loss of CD26.
Flow Cytometry
- More useful than Sezary prep
- Confirms presence of blood disease
- CD4+/CD7- or CD4+/CD26-
- False positive increase in CD4+/CD26- T-cells in atopic dermatitis
- > 20% circulating lymphocytes that are CD4+/CD26- highly suggestive of Sezary syndrome
Don’t forget about physical examination, i.e., palpate lymph nodes.
It’s important to remember that when you’re treating Sezary syndrome, you should NOT use:
- Cyclosporine
- Azathioprine
- Mycophenylate mofetil
- Anti-tumor necrosis factor agents
- Anti-IL-12/23
The reason that we cannot use the therapies mentioned above is because the cellular immune response is absolutely critical for these patients. Combined immune potentiators for advanced CTCL have been shown to produce high response rates. These include combinations of interferons, bexarotene (or an RAR specific retinoid), GM-CSF, and photopheresis. We also attempt to debulk the skin with PUVA or total skin electron beam.
It is noteworthy that a 2011 paper demonstrated that psoralen plus UVA light can be associated with the clearing of peripheral blood disease in advanced CTCL. (Raphael BA, et al. JAAD.2011;65(1):212-214.) In fact, three out of the five patients presented in this series cleared their blood when PUVA was added to their regimen. And we certainly can accomplish the same in the majority of patients who receive full dose total skin electron beam.
Why is the skin involved in T-cell lymphoma?
Malignant T-cells are very high expressors of cutaneous lymphoid antigen (CLA). When the cutaneous endothelium is activated by interferon gamma or tumor necrosis factor, you observe upregulation of E-Selectin. The cells then begin to adhere and roll very slowly. The malignant cells are also very high expressers of CCR4; this is important because CCR4 is the chemokine receptor for CCL17 (TARC) which is manufactured in the epidermis and which is responsible for attracting the cells into the skin.
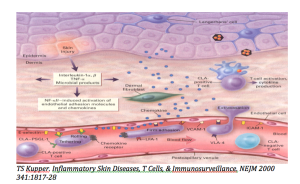
Phase I trial data demonstrate that KW-0761, an anti-CCR4 monoclonal antibody with enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) currently in phase III trials for leukemic forms of CTCL, is both efficacious and safe.
Treatment of CTCL
Alemtuzumab, which is particularly useful for leukemic CTCL in the absence of bulky nodal disease, targets CD52 on T-cells, B-cells, and monocytes, which at high dose, causes patients to become severly immunodepressed. In 2007, Maria Bernengo published a clinical protocol for the use of low-dose alemtuzumab.
- 3 mg days 1 and 3
- 10 mg days 5 and 7
- Continue 10 mg every other day until Sezary count < 1000/ mm3
- Monitor Sezary count Q2 weeks for one month then Q month
- If Sezary count exceeds 2000/ mm3 resume treatment
- High response rates of non-transformed disease
- Low infection rates
Alemtuzumab is extremely effective at this dose and Dr Rook has never seen a serious or life-threatening infection at his institution. But, nevertheless, patients still require prophylaxis against PCP, fungal infection, and against reactivation of Herpes and varicella zoster.
Histone deacetylase inhibitors are very effective for advanced disease, particularly romidepsin. Romidepsin is a parenteral agent that produces responses in advanced disease in about fifty percent for both tumor stage disease and refractory Sezary syndrome. It is generally pretty well tolerated unless the patient has serious cardiovascular disease. While vorinostat is easier to administer because it is oral, patients don’t tend to like it because of unpleasant side effects including diarrhea and fatigue.
Brentuximab vedotin is an agent that is likely to be useful for large-cell transformation and is under investigation. It is currently FDA-approved for CD30-positive lymphomas, ALCL, and relapsed Hodgkin’s disease. This drug is a fusion protein that contains an antibody directed at CD30. This is important because CD30 gets upregulated in large-cell transformation. Appended to it is monomethyl auristatin, a tubulin toxin. In view of its affinity for CD30, brentuximab can be administered to patients with refractory large-cell transformation. Adverse effects can include peripheral neuropathy.
Early Disease
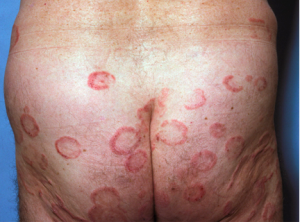
This is the typical arciform distribution of early disease. The fact that we can see clearing in the center is highly suggestive that the host immune response is playing a role in keeping this somewhat under control. The survival rate of patients with stage 1a disease when treated is similar to that of the general population. It is important to note that patients who present with plaques have a worse prognosis than patients who present with patches, these patients need to be treated vigorously and immediately.
Treatment of Stage T1 (patches or plaques on less than 10 percent of the skin surface area)
- Potent Topical Steroids
- Nitrogen Mustard (0.01-0.04% ointment)
- Carmustine (0.03-0.04% ointment)
- Narrowband UVB
- PUVA
- Topical Retinoids (Bexarotene, Tazarotene)
- Imiquimod
A novel mechlorethamine 0.016% gel (Valchlor) was FDA-approved in 2013 for the treatment of CTCL. Dr Rook advises that this compound be used with caution because it can be irritating. When using a topical chemotherapeutic, Dr Rook prefers to use it nightly in an effort to avoid drug resistance. However, because Valchlor is irritating, the recommendations are to use it every other night and perhaps in conjunction with topical steroids to prevent/reduce irritation.
Interferon Alpha and Antitumor Effects
Interferon activates anti-tumor cytolytic cells and inhibits the growth of malignant T-cells. It also inhibits the production of Th2 cytokines. We have also found that response rates are greater when used in combination with PUVA, retinoids, photopheresis, and/or interferon gamma. In order to minimize the side effects of interferon, Dr Rook utilizes a low-dose interferon. Nevertheless, there is a dose related effect with higher doses being more efficacious but are less well tolerated.
Treatment Protocol
- Initial Dose 5-2.5 million Units SQ 3 x per week
- Increase to: 5-7.5 million Units SQ 4-5 x per week
- Typical Dose: 5-5 million Units SQ every other day
Bexarotene
Bexarotene induces apoptosis of malignant T-cells. Keep in mind that not all patients will respond; there is only about a forty percent response rate due to the likely reason that the critical RXR receptors may not be expressed at the cell surface of the malignant population in all patients. Dr Rook recommends using bexarotene in combination with interferon, PUVA, and/or photopheresis in an effort to prevent resistance. If you are treating patients using bexarotene, they will require intensive control of hyperlipidemia, i.e, use of a statin and/or other lipid-lowering drugs. Bexartone also produces central hypothyroidism by suppressing TSH so thyroid function should be measured with free T4 (not TSH). Bexarotene may be more difficult to use in diabetics and patients with hyperlipidemia.
Imidazoquinolines
These particular drugs are powerful Toll-like receptor agonists and are therapeutically active for CTCL. Many patients may not respond to imiquimod. Dr Rook prefers to use the Zyclara Pump (3.75% Imiquimod) as it is easier to control the amount dispensed as well as easy to spread.
Resiquimod, a combined TLR 7 and 8 agonist, is at the first stages of the pipeline. It has a bioavailability ten times greater than imiquimod and potency up to 100 times greater than imiquimod. A one-gram application induces a systemic interferon alpha response. Dr Rook is conducting a phase 1 trial with resiquimod and has found that patients get systemic absorption, activation of circulating antigen-presenting cells, and widespread clinical responses. This appears to be a very exciting drug that clears treated lesions and induces the resolution of distant lesions.
MauiDerm News Editor-Judy Seraphine