Eruption! Pediatric Acne
Larry Eichenfield, MD
Dr Eichenfield provided the audience at MauiDerm 2014 with an update on the new pediatric guidelines for the management of acne. These guidelines, published in May of 2013, were developed by the American Acne and Roseacea Society and endorsed by the American Academy of Pediatrics, which is the first time that the Academy has a set of guidelines for the management of acne. One of the main reasons this was done was to address the practice gap between dermatologists and pediatricians. The group of experts was comprised of dermatologists with expertise in acne, pediatric dermatologists, and pediatricians.
As we know, acne ranges in terms of presentation and severity. Acne can be categorized by age, i.e., neonatal (0-6wk), infantile (0-1), mid-childhood (1-7), preadolescent (7-12), and adolescent (12-19).
Dr Eichenfield emphasizes that mid-childhood acne (age 2 – 7) acne is the most worrisome. It can be associated with premature adrenarche, Cushings Syndrome, CAH, gonadal/adrenal tumors, and precocious puberty. Mid-childhood acne is very uncommon, and is a sign of early adrenarche, often associated with a pathologic process. The assessment for mid-childhood acne includes assessment of testicular size (males), presence or absence of hirsutism, clitoromegaly, androgenetic alopecia, increased muscle mass, and/or deepening of the voice (males). Tests and examinations include a growth chart, evaluation including bone age, tanner stage, Total/Free testosterone, DHEAS, androstenedione, LH, FSH, prolactin, and 17OH-progesterone. The guidelines suggest that you should refer these patients to a pediatric endocrinologist.
What do the new guidelines say?
It’s important to know that pre-adolescent (7 ≤ 12 years) acne is common and may precede other signs of pubertal maturation. Work-up beyond history and physical is generally unnecessary unless there are signs of androgen excess, polycystic ovarian syndrome, or other systemic abnormalities. According to the data, there is evidence that acne, and possibly puberty, are now occurring at an increasingly earlier age for both males and females. If you look at some of the papers that were published about a decade ago, the amount of comedones that were seen in a ten or 12 year-old then is probably different now. Twelve is no longer an age point defining “normal acne;” if you are eight and above acne can be typical and common.
After the guidelines were published early acne in preteens was highlighted in several newspaper articles, and radio and television segments. Articles were published in both the New York Times and USA Today. In fact, after the New York Times article was published, there were over 157 blogs that provided an interesting perspective comparing what the general public thinks regarding acne and what we do as specialists. A recent analysis of the National Ambulatory Medical Care Survey (NAMCS) database assessed trends in the age of children seeking treatment for acne. NAMCS data from 1979 through 2007 were analyzed for all physician visits in which acne vulgaris was listed as a diagnosis in children aged 6 to 18. The analysis revealed a significant decrease in the mean age of children seeking treatment for acne over this period.
Over the past several years, clinicians have noted an earlier onset of acne—that is, the appearance of acne in patients as young as 8 or 9 years. Indeed, it has been suggested that 12 years of age should no longer be considered the low end of the “normal” range for the onset of acne.
Acne may be the first sign of the onset of puberty in children aged 7 to 11 years. The general clinical impression of earlier puberty is, in fact, supported by epidemiologic data in the US and elsewhere. In general, the trend toward earlier puberty is stronger among girls than among boys. In any event, the earlier onset of acne has mirrored the downward trend in puberty timing.
The guidelines provide an algorithm for the management of pediatric acne. If you can generally categorize the acne as mild, moderate or severe, you can access the algorithm. Keep in mind that this algorithm is slightly different than prior guidelines, in that for mild acne initial treatment benzoyl peroxide, based upon the evidence, made it as one potential, initial solo therapy. The guidelines can be found in the Journal of the American Academy of Pediatrics, or online at: http://pediatrics.aappublications.org/content/131/Supplement_3/S163.full.pdf.
Acne Guidelines: Highlights
The guidelines emphasize appropriate use of medications based upon disease severity. Oral antibiotics should be used concurrently with a topical retinoid because it is important to build a topical regiment to “transfer the patient to” after a limited course of antibiotics. A variety of studies show that 70 percent of the time you can transition your patient who is on an oral antibiotic and topical retinoid to a regiment of topical retinoid alone or combinations with topical antimicrobials (like benzoyl peroxide) and/or antibiotics
Topical retinoids may be used as monotherapy or in combination products and in regimens of care for all types and severities of acne in children and adolescents of all ages. It is important to remember that topical antibiotics are not recommended as monotherapy and if a topical antibiotic treatment is to be used for more than a few weeks, topical benzoyl peroxide should be added, or used in combination products. The guidelines also suggest that fixed-dose combination topical therapies may be useful in regimens of care for all types and severities of acne.
With regards to oral antibiotics, they are a reasonable approach for moderate to severe inflammatory acne vulgaris at any age. Tetracycline derivatives should not bee utilized in children 8 years of age and below. Second generation tetracyclines are “sometimes preferred” to tetracycline because of ease of use, fewer problems with absorption with food and minerals in vitamins and other supplements, and less frequent dosing. It’s very important that patients are educated and monitored for potential adverse events when utilizing oral antibiotics for acne.
Hormonal therapy is actually interesting because there are people who are very pro-hormonal therapy and others are a bit more conservative and prefer oral antibiotics. The group ended up stating that combined oral contraceptives (OCs) may also be useful as second-line therapy in regimens of care in pubertal females with moderate to severe acne; however, tobacco use and family history of thrombotic events should be assessed. Due to concerns about growth and bone density, many recommend withholding OCs for acne until one year after onset of menstruation.
Isotretinoin is recommended for severe, scarring, and/or refractory acne in adolescents and may be utilized in younger patients. Remember that extensive counseling, particularly regarding the avoidance of pregnancy, as well as careful monitoring of potential side effects and toxicities, is recommended. As far as the specific discussion on isotretinoin in pediatric use, there are bone mineralization changes; however, the data is inconsistent and it is not associated with increased factures. Hyperostoses are very uncommon for acne and there has been one case of premature epiphyseal closure in a patient on isotretinoin for acne, but that’s not necessarily attributable to the isotretinoin. IBD is controversial, but counseling is reasonable.
Practice Gaps
Dr Eichenfield states that there is a chasm in the way that General Practitioners, Pediatricians, and Dermatologists treat acne. A 2014 paper by Tan et al showed that topical retinoids were prescribed in 41 percent of acne visits. Older age, male gender and having Medicaid insurance were associated with a lower likelihood of receiving a topical retinoid prescription. Moreover, the researchers found that in the Medicaid dataset, patients who saw a pediatrician or general practitioner had lower odds of receiving a topical retinoid prescription versus those patients who saw a dermatologist.
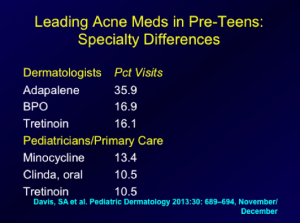
Another study looked at the National Ambulatory Medical Care Survey (NAMCS) data regarding the treatment of preadolescent acne in the United States. The data were stratified according to age group and physician specialty. The findings are presented below:
What are Dr Eichenfield’s thoughts on the practice gaps?
- Over-reliance on oral antibiotics
- Use of oral antibiotics without BP
- Use of oral antibiotics without Retinoid
- Use of topical and oral antibiotics together, without retinoid
- Under appreciation of early, significant acne as predictor of worse acne over time
Literature has shown that early comedonal acne may predict later severe acne. So remember that if you see a patient with a high number of comedone counts at an early age, their chances of severe inflammatory acne at a later age is much higher than someone who has low comedone counts early on.
Another issue that Dr Eichenfield is appreciating more and more in clinical practice is that you can have very early, subtle scarring. We know that there is a lot of scarring that occurs without nodular-cystic disease, so this is very common. This is an important to try to get patients evaluated early so that scarring can be prevented, and minimized. Dr Eichenfield advised that a useful technique is to side-light the face and look for depressions in the face, displaying scarring as opposed to post-inflammatory hyperpigmentation or persistent erythema.
A study by Patel and colleagues aimed to determine what types of acne lesions preceded the development of atrophic acne scars. Twenty-two patients with mild to moderate acne were enrolled in a split-face study in which one side was treated with non-ablative laser and the other remained untreated. A series of standardized digital facial photographs was obtained from the untreated side at 2-week intervals from baseline to week 12, and all photographs in the series were aligned with the baseline photo. When all of the atrophic scars were tracked to baseline, 53 were found to have arisen from clinically normal skin, 30 were established scars, and 21 arose from acne lesions, including closed comedones. However, no open comedones at baseline corresponded to atrophic scars. The results of this study not only verify that inflammatory acne lesions often lead to atrophic scarring, but also demonstrate that acne scars may arise from initially comedonal lesions, as well as from clinically normal skin. Moreover, they indicate that a period of 12 weeks is sufficiently long to develop and establish atrophic scars. Thus, aggressive treatment of both inflammatory and comedonal acne is warranted to minimize acne scarring.
What about Isotretinoin?
We know that isotretinoin is the most effective treatment for acne; however, the optimal dosing regimen is still unknown. Dr Eichenfield comments that he uses isotretinoin commonly. He also states that he tends to be biased towards lower-dose isotretinoin on a daily basis, working up to cumulative doses of 120-150 mg/kg. He commonly will the daily doeses up to the “highest comfortable dose,” that is, the highest dose with minimal significant side effects or laboratory abnormalities.
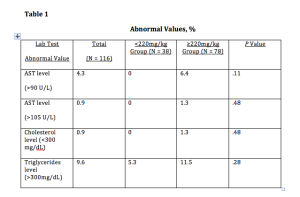
A recent publication looked at 180 patients with acne resistant to other treatments who were enrolled in an observational, prospective study of istotretinoin with cumulative doses less than to 220 mg/kg versus isotretinoin greater than or equal to 220 mg/kg. Of these patients, 116 participated in the 12-month follow-up survey. At that time, 97.4 percent of the patients reported that their acne was improved. Overall, acne in 32.7 percent of the patients in the study relapsed at 12 months, and 1.72 percent of the patients required a retrial. In the lower-dose treatment group, the relapse rate was 47.4 percent compared with 26.9 percent in the high-dose group. Almost 100 percent of the patients in both treatment groups developed cheilitis and xerosis during treatment. Retinoid dermatitis was significantly more common in the high-dose treatment group and none of the other adverse effects were significantly different between the two groups. However, it should be noted that in the higher dose group, nine patients had persistent muscle aches, eight patients had persistent joint aches, and two patients had hearing changes. (Blasiak RC et al. JAMA Dermatol 2013;149(12):1392-1398) Also of importance with regards to this study are the laboratory abnormalities based upon the dosing. (See table 1)
Dr Eichenfield states that this publication has yet to “move him” to abandon his current methodology with regards to isotretinoin dosing for this patients.
Idiopathic Facial Asceptic Granuloma (IFAG) and Childhood Rosacea
We have seen a very interesting change in perspective regarding this disease. Occasionally, we see these patients who present with lumpy, cystic-type lesions, separate from acneiform lesions. A multi-center study of four French dermatologic centers looked at patients who were diagnosed with IFAG between October 2000 and July 2007. Thirty-eight patients were included in the study. The median age at the time of diagnosis of IFAG was 43 months, with a median follow-up of 3.9 years. Sixteen patients (42.1%) had at least two criteria of childhood rosacea, 11 of 32 (34.4%) with a single lesion and 5 of 6 (83.3%) with multiple lesions. Remember that childhood rosacea presents with flushing, permanent or recurring erythema, papules and postules without comedones or microcysts, convexity predominance of lesions, ocular rosacea (chalazions, conjunctival hyperemia, keratitis). (Prey S, Ezzedine K et al. Pediatric Dermatology 2013;30:429-32)
What does this mean to us? Children with IFAG are at risk for childhood rosacea, and follow-up is advised, including periodic ophthalmologic assessment.
MauiDerm News Editor- Judy Seraphine