Infectious Diseases Update: Part 1: Interesting ID Literature
Ted Rosen, MD
In this presentation, Dr Rosen provides us with new information on infectious diseases that has recently appeared in the literature.
MRSA
A study in the International Journal of Antimicrobial Agents states that minocycline is often forgotten but preferred to trimethoprim-sulfamethoxazole or doxycycline for the treatment of community-acquired methicillin-resistant Staphylococcus aureus skin (MRSA) and soft-tissue infections. (Cunha B. Int J Antimicrob Agents. 2013;42:497). They point out that there is about a thirty percent discrepancy in discordance between the in vitro antibacterial testing and actual clinical effectiveness when it comes to the tetracycline family, this is less so with minocycline.
When treating MRSA, remember that incision and drainage is the most important intervention, if possible. CA-MRSA can be either modestly or severely virulent depending upon the presence or absence of PVL (Panton-Valentine leukocidin).
Typically we have some choices in treatment. We tend to leave linezolid for the last resort and clindamycin often has inducible resistance so we’re left with doxycycline, TMP-SMX, and minocycline. Cunha and his colleagues point out that second only to TMP-SMX, minocycline is the best in tissue penetrability and is equivalent to linezolid in predictable efficacy. Nationwide, only one to two percent of MRSA are resistant to linezolid and minocycline; however, up to twelve percent are resistant to doxycycline.
Because there is a generic minocycline available, the cost issues are largely negated. There are other issues such as hypersensitivity reactions and rare autoimmune phenomena. The authors feel, from a microbiological standpoint, minocycline is a preferred drug.
Herpes
We now have some new therapies available for the treatment of herpes. Herpotherm utilizes thermotherapy for genital herpes. A prospective study conducted in Russia evaluated 32 women. Twenty-one patients received thermotherapy plus acyclovir and ten received thermotherapy alone. Treatment was initiated with the first objective sign of HSV-2. Within one day, symptoms were gone or almost gone, with or without acyclovir as concomitant therapy demonstrating that thermotherapy is beneficial for genital herpes. Although exposure id of short duration (seconds), patients should be warned to expect some discomfort.
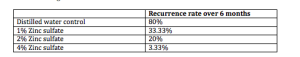
Topical zinc sulfate in-vitro inhibits the growth of HSV. Mahajan and colleagues tried various concentrations of zinc sulfate on 100 clinical and Tzanck verified men with genital HSV over a six-month period. They had a very specific treatment protocol:
- Q5d x 1 mo
- Then Q10d x 2 mo
- Then Q15d x 3 mo
Of note, all of the patients had nine or more recurrences per year, i.e., frequent outbreaks of genital HSV. Here’s what the authors found:
4% Zinc sulfate is a cheap, non-toxic therapy that is easy to use and may be a viable treatment for hard to manage HSV-2. The key is to apply this when there is an outbreak and as it heals, patients should continue to follow the protocol. (Mahajan BB. Indian J Sex Transm Dis. 2013;34:32-34.)
Flat Warts
A study by Li and colleagues, published in the Journal of the European Academy of Dermatology and Venereology evaluated photodynamic therapy with five percent, ten percent, and twenty percent aminolevulinic acid (ALA) in the treatment of generalized recalcitrant facial verruca plana. This was a prospective study of 55 adult patients with bilateral facial flat warts. The two sides of the patient’s faces were randomized to receive ALA 5 percent, 10 percent or 20 percent. There was no placebo control. Patients were irradiated with a 633-nm red light in two 7.5- minute sessions, separated by 30 minutes of non-exposure. This was repeated three times, every two weeks. This study demonstrated that ALA 10 and 20 percent were most efficacious for the treatment of generalized recalcitrant facial verruca plana. In the United States, we have Levulan Kerastick (20% ALA-d). (Li Q, et al. J Eur Acad Dermatol Venereol. 2013 Nov 25. [Epub ahead of print])
Onychomycosis in Psoriatic Patients
A systematic review by Klaassen and colleagues analyzed the literature with regards to the prevalence of onychomycosis in patients with nail psoriasis. They looked at over 700 studies out of which they chose ten based on their criteria. The authors found that an average of 18 percent of psoriasis patients with nail dystrophy have concurrent onychomycosis. There was no shift from dermatophytes to saprophytes or yeast in these studies. Because psoriasis is treated with immunosuppressive agents, this may aggravate any concurrent fungal infection. (Klaassen KMG, et al. J Eur Acad Dermatol Venereol. 2013 Aug 19)
Take Home Message: It is important that we check for fungal disease in patients with psoriasis nail dystrophy before instituting therapy.
Atypical Mycobacteria/Nontuberculous Mycobacteria
In a case series and epidemiologic study, Falsey and colleagues looked at cutaneous inoculation of nontuberculous mycobacteria during professional tattooing. How does this happen? One to four weeks after the tattoo is placed, it may itch or hurt and there are monomorphous crusted papules or postules that are confined to the tattoo area. At first blanche, we might look at this and think it’s bacterial, but it turns out that it is Mycobacterium either abcessus or chelonae. Clarithromycin, minocycline and ciprofloxacin are all viable treatment options; however, the authors strongly recommend that you send off one the papules for culture and sensitivity because the sensitivities vary widely. Why does this happen? Because the atypical mycobacteria are found as a contaminant in the tattoo ink or sometimes the non-sterile water used to dilute the ink (especially when outsourced from China). (Falsey R, et al. Clin Infect Dis. 2013;57(6):e143-147.)
Take Home Message: Think NTM in new tattoo lesions
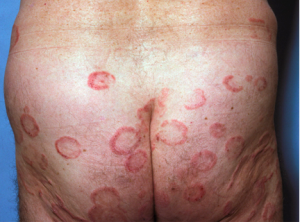
Herpes Zoster
Wang and colleagues conducted a population-based retrospective cohort study looking at herpes zoster infection and acute coronary syndrome (ACS), i.e., any event that suddenly decreases blood flow to the heart muscle (heart attack, unstable angina). This study included 59,958 patients with herpes zoster compared to an age/sex- matched cohort of 231,832 non-zoster patients. The authors found that acute coronary syndrome was associated with herpes zoster 25 percent more commonly. This was statistically significant (p=.0001) at both three months and one year after the herpes zoster was resolved. (Wang CC, et al. Br J Dermatol. Dec 6, 2013. e-pub ahead of print)
Take Home Message: Warn zoster patients about the risk of ACS and review symptoms of ACS.
Flood-related Skin Diseases
Flood, one of the most common natural disasters, has contributed to many public health problems. A study conducted in Thailand identified the following flood-related dermatological disorders:
- Irritant contact dermatitis
- Webspace tinea pedis
- Webspace mixed infection
- Cellulitis (S. pyogenes, Aeromonas)
- Gas gangrene (clostridium)
- Fasciitis (Vibrio spp)
- Traumatic wounds (check cuts for lacerations, punctures, serious digital injuries)
- Insect bites and stings (mosquito, fire ant, centipede)
- Animal bites (stray dogs/cats, snakes)
- Aggravation of pre-existing skin disease due to psychic trauma (psoriasis, atopy/eczema, alopecia areata, urticaria, vitiligo)