TNF-a Inhibition and the Treatment of Hidradenitis Suppurativa
Bruce Strober, MD, PhD
In this presentation, Dr Strober provides us with new data on TNF alpha inhibition in the treatment of hidradenitis suppurativa (HS). HS is sometimes referred to as acne inversa; however, Dr Strober feels it is a different disease based upon a lot of lines of evidence.
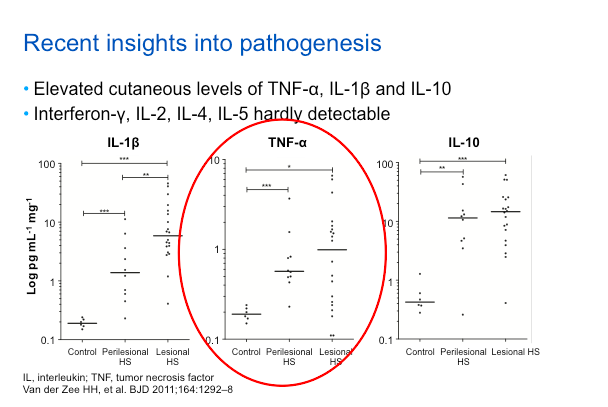
Based on recent insights into the pathogenesis of this disease, TNF inhibition has found to be an effective treatment. Essentially, it’s phenomenological, because if you look at lesional skin, there is more TNF alpha as compared to peri-lesional as compared to control skin. This isn’t to say that TNF is the only relevant cytokine; in fact, it may be the first of many which will be targeted over the next decade. Just like in psoriasis, TNF will be the first cytokine studied for targeting in HS.
Etanercept
Overall, etanercept (ETN), at the doses that have been studied, does not appear to be effective treatment for HS. The prospective, double-blind, placebo-controlled trial of 20 subjects were randomized 1:1 for ETN 50mg BIW versus placebo for 24 weeks. The primary efficacy endpoint assessed the Physician Global Assessment (clear or mild). Secondary endpoints included change in Patient Global Assessment and quality of life (DLQI). The results demonstrated that there was no statistically significant difference in benefit between ETN and placebo. There are some case reports that also verify this phenomenon.
Infliximab
The case reports for infliximab, which were published several years ago, showed efficacy in the treatment of HS; however, many patients had concomitant IBD, which is not uncommon among patients with HS.
A prospective, double-blind, placebo-controlled trial of infliximab IFX) included 38 subjects randomized to either IFX or placebo. The study’s primary endpoint was a 50 percent reduction in HS Severity Index (HSSI) at week 8 and secondary endpoints included change in Visual Analog Scale (VAS) and DLQI.
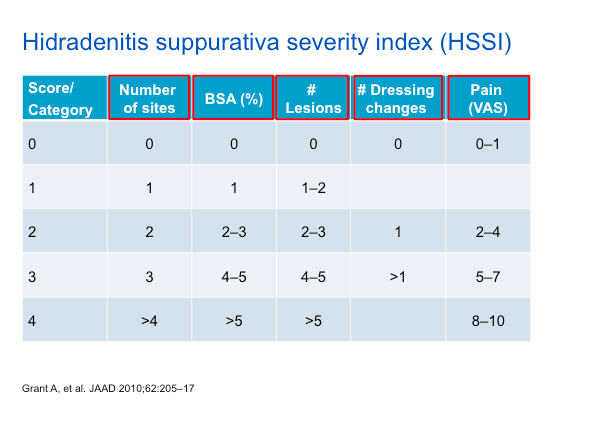
As noted in the table above, the HSSI score takes into account a variety of features, including dressing changes and pain—which are aspects of the HS experience that should be queried with each patient.
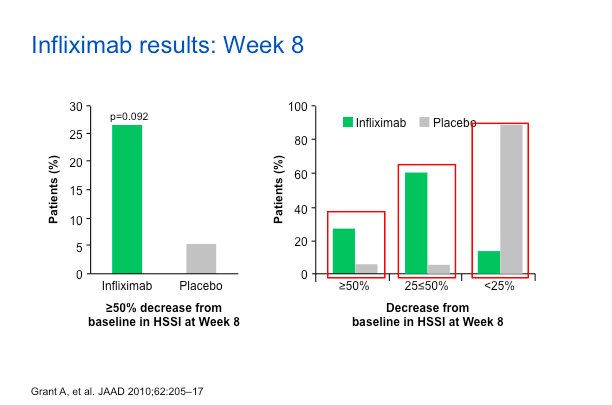
As we can see, about 25%-30% of patients on IFX have a reduction in HSSI score versus the placebo group. If you look at the 25%-50% reduction range, you can still see a bias towards IFX. Dr Strober feels that these are good data substantiating the value of IFX for the treatment of HS.
The VAS score as well as DLQI also show that IFX did better than placebo.
Dr Strober has used IFX on at least forty patients and feels that it really is the best drug out there, if you can access it. He also recommends using it with methotrexate to sustain the effect of IFX.
Adalimumab
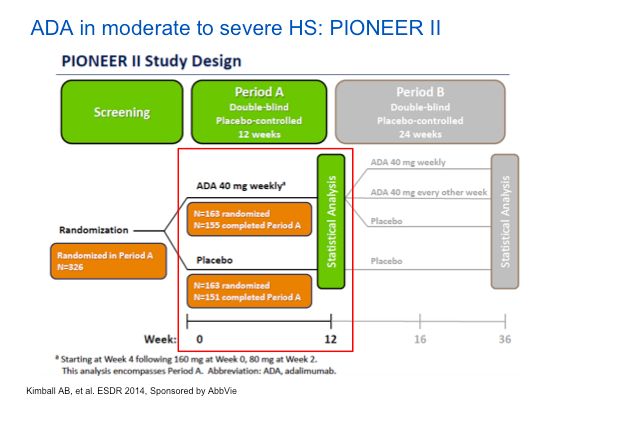
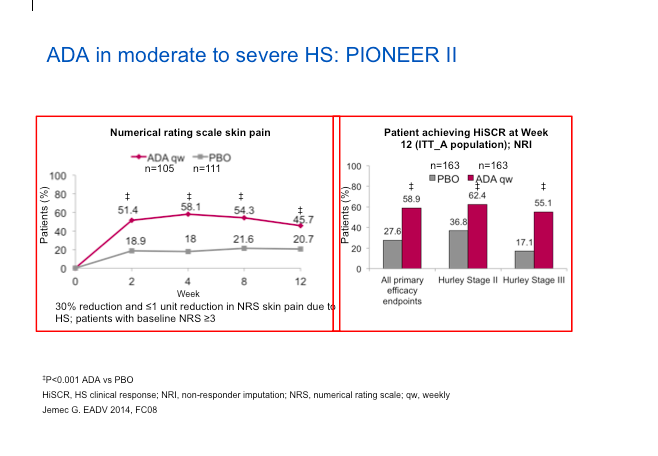
Adalimumab (ADA) represents the best set of studies done to date for the treatment of HS. The PIONEER II study looked at the safety and efficacy of ADA versus placebo in patients with moderate-to-severe HS after treatment for the first 12 weeks. The endpoint involved counting abscesses and inflammatory nodules. The HiSCR score, which Dr Strober feels is a valid score, is not dissimilar from other endpoints, in that it measures relevant issues in HS.
The study design was rigorous, in that they did a randomized, placebo-controlled approach. They key point here is that ADA will be dosed weekly, not every other week as it is when treating psoriasis. The phase II studies showed that ADA every other week did not perform as well.
Interestingly, many more women were enrolled in this study than men. Also of note, is that two thirds of the patients in the study were smokers. Remember that HS is a systemic, inflammatory disease. Much like psoriasis, we should think of it as a systemic syndrome of inflammation.
When we look at the data (below), we can see the numerical improvement in the rating scale of skin pain is biased towards ADA versus placebo and we can see more people achieving a HiSCR on ADA versus patients on placebo.
The ADA group also does better with regard to inflammatory lesion count reduction as well as the Sartorius Score reduction.
The safety data are remarkably clean in these studies, particularly focusing on infection, serious infection and malignancy. If anything, there were more events in the placebo group.
Clinical Pearls
What do we need to remember about HS?
- HS is a chronic, negatively life-impacting disease.
- Associated with metabolic syndrome (obesity, DM, HTN, dyslipidemia)
- Pathogenesis multifactorial; primarily inflammatory and not infectious, and our understanding is evolving
- Phase II/III studies with adalimumab are the best studies, to date, and convincing of efficacy and good safety
- Infliximab also is very effective
MauiDerm News Editor-Judy L. Seraphine, MSc