Update on Psoriatic Arthritis and the Focus on New Treatments
Based on the Maui Derm 2018 presentation by Arthur Kavanaugh, MD, Professor of Medicine and Director of the Center for Innovative Therapy (CIT) at University of California in San Diego, California
Article by Jo Ann LeQuang and the Journal of Clinical and Aesthetic Dermatology
Psoriasis is the most prevalent auto-immune disease in the United States, with 2.2 percent of the population affected (7.5 million), of whom approximately 10 to 30 percent will develop psoriatic arthritis. Psoriatic arthritis can develop at any age but onset typically occurs between the ages of 30 and 50 years.72 The clinical presentation of psoriatic arthritis varies widely among patients, and, to this end, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) established six domains for treatment of psoriatic arthritis (not all of which affect each patient): peripheral arthritis, axial disease, enthesitis, dactylitis, skin disease, and nail disease.73 Since patients with psoriatic arthritis have different clinical presentations with different domains affected, an individualized therapeutic approach is required. Periodic re-assessment is necessary as psoriatic arthritis is a chronic, progressive condition. Further complicating the situation is that psoriatic arthritis, like plaque psoriasis, is associated with numerous comorbidities, including mental health disorders (depression, anxiety), obesity, Type II diabetes mellitus, osteoporosis, cardiovascular conditions, and cancer.74
The pharmacoeconomic implications of psoriatic arthritis. In a national cohort study from Denmark,75 10,525 patients with psoriatic arthritis and 20,777 matched controls were evaluated for employment, comorbidities, and costs to the healthcare system and society. Patients with psoriatic arthritis had significantly more comorbid conditions than controls, including cardiovascular disease, respiratory disease, and infectious disease, higher total healthcare costs, and lower incomes than controls. The relative risk (RR) of disability for patients with psoriatic arthritis progressed from an RR of 1.36 at five years prior to diagnosis (95% CI, 1.24–1.49) to an RR of 1.60 (95% CI, 1.49– 1.72) at the time of diagnosis to an RR of 2.69 10 years following diagnosis (95% CI, 2.40–3.02).75 A study in the United States comparing 5,492 matched pairs of patients with psoriatic arthritis and controls found the most common comorbidities were hyperlipidemia, hypertension, and diabetes and that patients with psoriatic arthritis had significantly more prescriptions, inpatient admissions, emergency department visits, and outpatient visits than controls and significantly higher total costs, pharmacy costs, and medical costs than controls.76 The total annual estimated healthcare burden of psoriasis overall could be over $35 billion (including direct costs plus lost productivity).77 The severity of psoriasis and psoriatic arthritis increases the cost.78
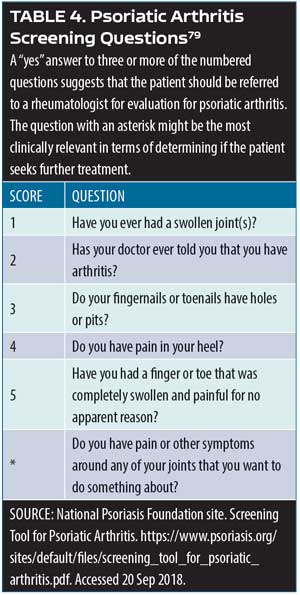
Screening psoriasis patients for psoriatic arthritis. Patients with psoriasis should be assessed periodically for joint pain, as it is a characteristic symptom of psoriatic arthritis. A simple assessment tool involves the use of a patient diagram (which can be an outline of human figure) where patients can mark the particular areas that cause them pain.79 Ibrahim and colleagues have defined five fundamental questions to be used in this assessment following identification of joint pain sites (Table 4).79 An important additional question to this screening instrument is: Do you have pain or other symptoms around any of your joints that you want to do something about? Clinicians might also wish to consider other psoriatic arthritis symptoms in this evaluation, such as joint swelling or tenderness, pain on movement, loss of function, and limitations to range of motion.
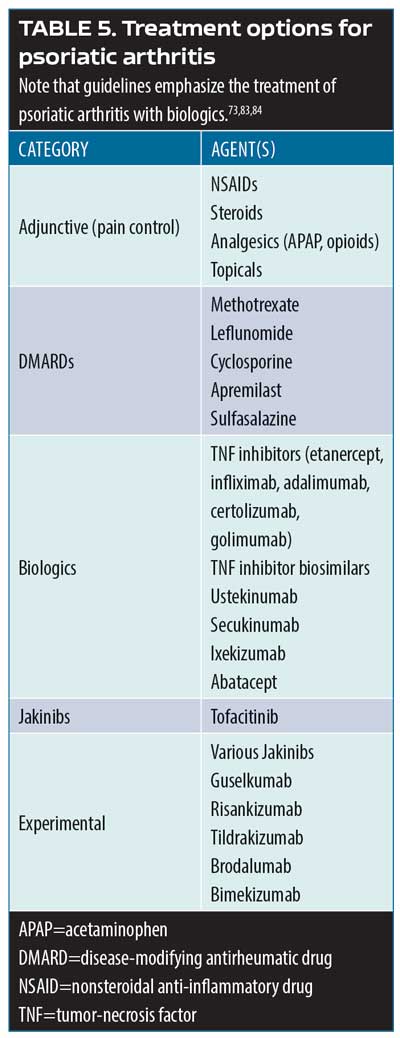
Psoriatic arthritis treatment options. Over 50 investigation monoclonal antibodies are being currently evaluated in late-stage clinical trials, and it is anticipated that at least half a dozen could be approved for market release in the coming year.80 The most important new developments in psoriatic arthritis involve these and other new treatment options. The main cytokine targets for the treatment of psoriatic arthritis are TNF, IL- 22/23, IL-17, and jakinibs (Table 5).81–84 Biologic agents serve to interrupt systemic inflammatory pathways.85
Golimumab. Golimumab is a human monoclonal antibody and TNF inhibitor that targets IL-6. In a Phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial (GO-VIBRANT),83 480 patients were randomized to two groups: one received intravenous (IV) placebo (n=239) and the other IV golimumab at 2mg/kg (n=241) at Weeks 0, 4, 12, and 20. American College of Rheumatology 20-percent improvement criteria (ACR20) at 14 weeks was the primary endpoint and achieving at least PASI75 at 14 weeks was among the secondary endpoints. By Week 14, 75.1 percent of the patients in the golimumab group had achieved ACR20 versus 21.8 percent of the patients in the placebo group (p<0.001); at this time, 59.2 percent of the golimumab group compared with 13.6 percent of the placebo group had met the PASI75 endpoint (p<0.001). Adverse events were similar between groups, with 40.6 percent of placebo group and 46.3 percent of golimumab group having had at least one adverse event over the course of 24 weeks. The most commonly reported TEAE was infection (20.0% in the golimumab group compared with 13.8% in placebo group). No opportunistic infections or tuberculosis were reported in the study. Infusion site reactions were mild and rare (<2%). Discontinuation due to an adverse event was similar between groups, with 1.3 percent of the placebo group and 2.1 percent of the golimumab group leaving the study. Two patients died during the course of the study, both of whom were in the placebo group.86
The GO-VIBRANT study was preceded by the GO-REVEAL study,87 which found that patients with psoriatic arthritis exhibited greater improvement in signs and symptoms at 14 weeks with subcutaneous golimumab 50mg or 100mg versus placebo. ACR20 was achieved at 14 weeks by 48 percent of all patients in the golimumab group (51% of the 50mg and 45% of the 100mg group) versus nine percent of the patients in the placebo group (p<0.001). In this study, 74 percent of patients had at least three-percent BSA involvement with psoriasis at baseline; at 14 weeks, 40 percent of the golimumab 50mg group, 58 percent of golimumab 100mg group, and three percent of the placebo group achieved PASI75 (p<0.001). Golimumab was well-tolerated by patients.87
TNF-inhibition persistence. In five-year registry of patients with a rheumatologist’s diagnosis of psoriatic arthritis at the point they received their first TNF inhibitor, 46.7 percent of these patients were still taking the initially prescribed TNF inhibitor at five years. Factors associated with better five-year persistence were male sex, the use of etanercept or adalimumab rather than infliximab, and the lack of comorbid conditions at baseline. Estimates of five-year persistence for the first, second, and third TNF inhibitor were 53 percent (range 49–57%), 60 percent (range 43– 57%), and 48 percent (range 36– 59%), respectively.88 This suggests that there is good long-term persistence with TNF inhibition therapy.
Subcutaneous secukinumab. Secukinumab is a human monoclonal antibody that selectively targets IL-17A. In a randomized, double-blind, parallel-group clinical study (FUTURE 5),89 996 patients with psoriatic arthritis were randomized to receive either 300mg subcutaneous (SQ) secukinumab with a loading dose (n=222), SQ secukinumab 150mg with a loading dose (n=220), SQ secukinumab 150mg without a loading dose (n=222), or placebo (n=332) over 24 weeks. At 16 weeks, ACR20 response was achieved by 62.6 percent of patients in the secukinumab 300mg group, 55.5 percent of patients receiving secukinumab 150mg with loading dose, 59.5 percent of patients in the group who received secukinumab 150mg without loading dose, and 27.4 percent of patients in the placebo group (p<0.0001 for all doses compared to placebo). ACR50 and ACR70 response rates were significantly higher at Week 16 for all secukinumab groups compared to placebo. The rate of adverse events was similar across groups (any secukinumab group 56.3% vs. placebo 62.0%; the highest rate of adverse events occurred in the secukinumab 300mg group at 63.1%). The most frequently reported adverse events were upper respiratory tract infection, dyslipidemia, headache, hypertension, diarrhea, hypercholesterolemia, and urinary tract infection. However, most of these adverse events were single events. Results from FUTURE 5 suggest that secukinumab delivered with a loading dose and maintenance dose or at high doses of 300mg more significantly reduced joint damage associated with psoriatic arthritis than placebo or secukinumab without a loading dose. In fact, the 300mg dose of secukinumab provided numerically improved responses compared to the 150mg secukinumab dose with or without a loading dose.89
Apremilast. Apremilast is a phosphodiesterase-4 inhibitor that was shown to be effective in the treatment of psoriatic arthritis in the Psoriatic Arthritis Long-Term Assessment of Clinical Efficacy (PALACE) Phase III pivotal clinical trials (PALACE-1, PALACE-2, and PALACE-3).90 In all three PALACE studies, ACR20 was achieved at 16 weeks by significantly more patients taking apremilast 20mg or 30mg twice a day (BID) than placebo, with results durable out to 52 weeks. Apremilast was generally well tolerate by patients with the most commonly reported adverse events being diarrhea and nausea. In a meta-analysis of apremilast in patients with psoriatic arthritis,91 significantly more patients in the apremilast group achieved ACR20 at 16 weeks versus placebo. Data from the PALACE-1 trial has been collected for five years and grouped by patient response (ACR20, ACR50, and ACR70). At five years, 28.4 percent of patients achieved ACR70 and 46.9 percent achieved PASI75. Results are durable and trend toward increasing improvement.
Risankizumab. Risankizumab is a human monoclonal antibody that targets the p19 subunit of IL-23 and, in that way, indirectly inhibits the IL-23 pathway. In 2017, a Phase II study by Papp and colleagues92 reported that selective IL-23 blockade with risankizumab resulted in a superior clinical response in patients with moderate-to-severe plaque psoriasis (not psoriatic arthritis) compared with ustekinumab. In a Phase II study of 185 psoriatic arthritis patients,93 subcutaneous risankizumab 150mg and 75mg were compared to placebo. Patients were stratified by prior use of TNF inhibitors and concurrent methotrexate use. In this study, 49.4 percent of patients had skin psoriasis of three percent or greater BSA. At 16 weeks, significantly more risankizumab patients achieved ACR20 (57.1–65.0%) versus placebo (37.5%), and significantly more patients in the risankizumab group achieved PASI75, PASI90, and PASI100 than patients in the placebo group. TEAEs were similar among groups with the most frequently reported side effect being infection; no deaths and no cases of tuberculosis were reported. One MACE occurred in the risankizumab group.93
Tofacitinib. Tofacitinib is a Janus kinase inhibitor that was evaluated in a Phase III non-inferiority clinical study in 1,106 patients with moderate-to-severe plaque psoriasis.94 Patients were randomized to receive either tofacitinib (5mg or 10mg BID) or etanercept (50mg twice weekly). Tofacitinib 10mg BID was non-inferior to etanercept 50mg twice weekly; the same did not apply to tofacitinib 5mg BID. In this 12-week study, 58.8 percent of the etanercept group achieved PASI75 compared to 63.6 percent of the tofacitinib 10mg BID group, and 39.5 percent of the tofacitinib 5mg BID groups achieved PASI75 at 12 weeks versus 5.6 percent of the placebo group.94
A 12-month, double-blind, active- and placebo-controlled Phase III trial compared tofacitinib, adalimumab, and placebo among 317 patients with psoriatic arthritis who had an inadequate clinical response to DMARD therapy.95 Patients were randomized to oral tofacitinib 5mg BID, oral tofacitinib 10mg BID, SQ adalimumab 40mg every two weeks, or placebo with a blinded switch at three months to tofacitinib 5mg or 10mg. ACR20 response rates at three months were 50 percent and 61 percent for the tofacitinib 5mg and 10mg groups, respectively, compared to 33 percent for the placebo group and 52 percent for the adalimumab group. Adverse event rates for 12 months were 66 percent and 71 percent in the tofacitinib 5mg and 10mg groups, respectively, 72 percent in the adalimumab group, and 69 percent and 64 percent in the placebo groups switched at three months to tofacitinib 5mg and 10 mg, respectively. Thus, tofacitinib was shown to be significantly superior to placebo at three months in patients with psoriatic arthritis who had failed treatment with a disease-modifying antirheumatic drug (DMARD) treatment.
Psoriatic arthritis comorbidities. Psoriatic arthritis, like plaque psoriasis, is associated with numerous, potentially serious comorbid conditions that can lead to decreased function, complicate treatment, and reduce the patient’s quality of life. Among these comorbidities are obesity, infections, metabolic syndrome, Type II diabetes, inflammatory bowel disease, autoimmune ophthalmic disease, osteoporosis, cancer, fatty liver disease, kidney disease, cardiovascular disorders, and cancer.96 A comparison of patients with psoriatic arthritis (n=90) and 240 controls was made using coronary computed tomography angiography (CCTA) to evaluate atherosclerosis. Patients had no prior history or known cardiovascular disease; they underwent CCTA for chest pain or because they had risk factors for heart disease. Patients with psoriatic arthritis had an increased prevalence, greater burden, and more severe coronary atherosclerosis on CCTA than control patients. The prevalence of overall plaque in the coronary artery was 60 percent versus 35 percent (p<0.001) for psoriatic arthritis versus control groups. For calcified plaque, it was 32 percent versus 17 percent (p=0.002) for patients with psoriatic arthritis versus control patients; for mixed plaque 22 percent versus eight percent (p<0.001), for non-calcified plaque 43 percent versus 22 percent (p<0.001), and for combined mixed and non-calcified plaque 51 percent versus 26 percent (p<0.01). Three-vessel disease occurred significantly more frequently among patients with psoriatic arthritis than controls (13% vs. 3%, p<0.001) as did obstructive plaques (defined as >50% stenosis), which occurred in nine percent of patients with psoriatic arthritis versus three percent of control patients (p=0.033).97
References
72. National Psoriasis Foundation site. Statistics. Psoriatic Disease 2018. https://www.psoriasis.org/content/statistics. Accessed May 8, 2018.
73. Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016 May;68(5):1060–1071
74. Husni ME, Mease PJ. Managing comorbid disease in patients with psoriatic arthritis. Curr Rheumatol Rep. 2010;12(4):281–287.
75. Kristensen LE, Jorgensen TS, Christensen R, et al. Societal costs and patients’ experience of health inequities before and after diagnosis of psoriatic arthritis: a Danish cohort study. Ann Rheum Dis. 2017;76(9):1495–1501.
76. Feldman SR, Zhao Y, Shi L, et al. Economic and comorbidity burden among moderate-to-severe psoriasis patients with comorbid psoriatic arthritis. Arthritis Care Res (Hoboken). 2015;67(5):708–717.
77. Evans C. Managed care aspects of psoriasis and psoriatic arthritis. Am J Manag Care. 2016;22(8 Suppl):s238–243.
78. Burgos-Pol R, Martinez-Sesmero JM, Ventura-Cerda JM, et al. The cost of psoriasis and psoriatic arthritis in 5 European countries: a systematic review. Actas Dermo-Sifiliograficas. 2016;107(7):577–590.
79. Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27(3):469–474
80. Reichert JM. Antibodies to watch in 2017. mAbs. 2017;9(2):167–181.
81. Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72(3):436–439 e431.
82. Papp K, Pariser D, Catlin M, et al. Phase 2a randomised, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus Kinase (JAK) inhibitor, in patients with moderate to severe psoriasis. Br J Dermatol. 2015;173(3):767–776.
83. Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):
499–510.
84. Smolen JS, Braun J, Dougados M, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73(1):6–16.
85. Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 2016 ;137(1):
19–27.
86. Kavanaugh A, Husni ME, Harrison DD, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through Week 24 of the GO-VIBRANT Study. Arthritis Rheumatol. 2017;69(11):
2151–2161.
87. Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheumatol. 2009;60(4):976–986.
88. Fagerli KM, Kearsley-Fleet L, Watson KD, et al. Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis. Data from the British Society for Rheumatology Biologics Register. RMD Open. 2018;4(1):e000596.
89. Mease P, van der Heijde D, Landewe R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77(6):890-–97.
90. Mease PJ. Apremilast: a phosphodiesterase 4 inhibitor for the treatment of psoriatic arthritis. Rheumatol Ther. 2014;1(1):1–20.
91. Qu X, Zhang S, Tao L, Song Y. A meta-analysis of apremilast on psoriatic arthritis long-term assessment of clinical efficacy (PALACE). Exp Rev Clin Pharmacol. 2016;9(6):799–805.
92. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. New Engl J Med. 2017;376(16):1551–1560.
93. Mease PJ, Kellner H, Morita A, et al. Efficacy and safety results from a Phase 2 trial of risankizumab, a selective IL-23p19 inhibitor, in patients with active psoriatic arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-results-from-a-phase-2-trial-of-risankizumab-a-selective-il-23p19-inhibitor-in-patients-with-active-psoriatic-arthritis/. Accessed September 27, 2018.
94. Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561.
95. Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. New Engl J Med. 2017;377(16):1537–1550.
96. Ogdie A, Schwartzman S, Eder L, et al. Comprehensive treatment of psoriatic arthritis: managing comorbidities and extraarticular manifestations. J Rheumatol. 2014;41(11):
2315–2322.
97. Shen J, Wong KT, Cheng IT, et al. Increased prevalence of coronary plaque in patients with psoriatic arthritis without prior diagnosis of coronary artery disease. Ann Rheum Dis. 2017;76(7):1237–1244.