New Drugs and Therapies for 2016: Atopic Dermatitis
Drs. Neal Bhatia and Ted Rosen
Part 3 of an 8-part series on the large number of new topical and systemic medications that have become available or moved closer to approval in the last 12 months.
Approximately 18-25 million people in the United States suffer from atopic dermatitis, and 80-90% have mild or moderate disease. Patients suffering with the condition often try multiple treatments to treat their atopic dermatitis, yet many are not satisfied with the effectiveness of their medications.
Crisaborole 2% ointment
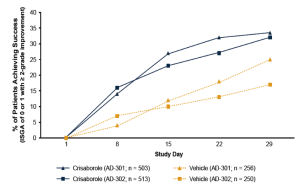
Crisaborole topical ointment, 2% is a novel, boron-based small-molecule phosphodiesterase (PDE) 4 inhibitor with anti-inflammatory properties. It has been evaluated in two phase III studies that involved more than 750 patients each. In these studies, patients were randomized to crisaborole or vehicle twice daily for a total of 28 days. Treatment was considered successful if on the 29th day a patient is gauged with an Investigator Global Severity Assessment (ISGA) score of 0 (clear) or 1 (almost clear), with a minimum improvement of two points from baseline. This outcome was achieved for about 33% of patients who received crisaborole and 17% of those treated with placebo (Figure 3). The most common side effects were pain at the application site and upper respiratory tract infections. A long-term study is being conducted to further evaluate safety with intermittent use of the medication for up to a year. If approved, crisaborole has the potential to offer physicians and patients a new, important therapeutic choice for treating mild-to-moderate atopic dermatitis.
Dupilumab
Dupilumab is a monoclonal antibody that blocks the actions of interleukin (IL) – 4 and IL-13. It is being developed by Regeneron and Sanofi for the treatment of atopic dermatitis and asthma. It has been evaluated in a 12-week phase IIa study and a 16-week phase IIb dose-ranging trial. Results from the 12-week study indicated that 85% of patients in the dupilumab group, as compared with 35% of those in the placebo group, had a ³50% reduction in the Eczema Area and Severity Index (EASI) score (EASI-50); and 40% of patients in the dupilumab group, as compared with 7% in the placebo group, had a score of 0 to 1 (indicating clearing or near-clearing of skin lesions) on the IGA. Pruritus scores decreased (indicating a reduction in itch) by 55.7% in the dupilumab group vs 15.1% in the placebo group. Results from the dose-ranging study indicated that dupilumab 300 mg once a week, 300 mg every 2 weeks, 200 mg every 2 weeks, 300 mg every 4 weeks, and 100 mg every 4 weeks were all significantly superior to placebo for decreasing EASI scores. The most frequent adverse event for dupilumab was nasopharyngitis.