Psoriasis: Update on Emerging Systemic Therapies
Craig Leonardi, MD
At MauiDerm 2014, Dr Craig Leonardi, Clinical Professor of Dermatology at Saint Louis University, presented us with the latest data on emerging systemic therapies for the treatment of psoriasis. According to Dr Leonardi, “the psoriasis space is going to move very quickly over the next year/year and a half…”
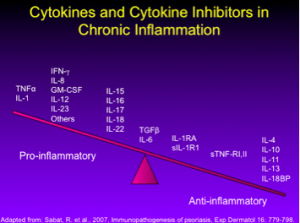
Dr Leonardi comments that many of our 2nd generation biologics were developed with these cytokines in mind (see below).
Remember that drugs come and go and many fall by the wayside; what’s important to us, as practitioners, is finding the best medicine for our patients.
Certolizumab Pegol (CZP)
CZP is a peglyated Fab fragment. It was initially approved for Crohn’s disease and subsequently approved for the treatment of rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. In 2005, there was a large phase II psoriasis trial of 176 patients with 10 percent BSA and PASI 12. It was conducted in Germany and France to look at CZP for the treatment of psoriasis. There were three treatment arms: 200mg sc qeow, 400mg sc qmonth, and placebo. PASI 75 results demonstrated 75 percent, 83 percent and 7 percent, respectively. PGA results (clear-almost clear) showed 53 percent, 72 percent and 2 percent, respectively among all treatment arms. There were no unexpected safety issues. This data tell us that CZP is both efficacious and safe for the treatment of moderate to severe plaque psoriasis. The fact that it is approved for PsA is also important to patients with PsO/PsA. This data was presented in 2007 at the American Academy of Dermatology and published as a paper in 2012. It appears that currently, UCB, the maker of CZP, has again taken an interest in this medication, as there is some development effort and we should be seeing a psoriasis trial quite soon.
Targeting the IL-12/23 Pathway
Both ustekinumab (IL-12) and briakinumab (IL-23) block the shared p40 subunit. When you block IL-12, you down-regulate a set a cytokines from the Th1 pathway, including INFy, IL-2 and TNF-alpha. When you block IL-23, you down-regulate IL-17 alpha, IL-17f, IL-6, TNF-alpha, IL-21 and IL-22.
Function of Th17 Effector Cytokines
IL-17a
•Expressed by memory NK and T cells
•Increased in psoriatic skin
•Subcutaneous injection à neutrophilia
•Enhances inflammation
•Enhances angiogenesis
IL-22
•Expressed in high levels by Th17 cells
•Increased in psoriasis (skin and plasma)
•Levels correspond to disease activity
•Induces keratinocyte hyperproliferation (in vivo, in vitro)
•Stimulates keratinocytes to secrete antimicrobial peptides
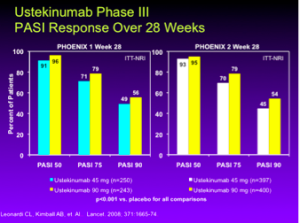
Ustekinumab is a high-performing drug for psoriasis patients. The data at week 28 in both the PHOENIX 1 and PHOENIX 2 studies, show about 70-79% of patients are achieving a PASI 75; this is a huge achievement for these patients.
New Development Efforts
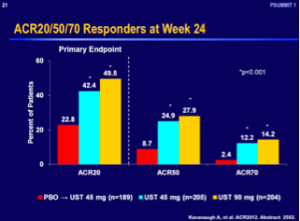
Ustekinumab was recently approved for the treatment of PsA, based upon its efficacy data with regards to an ACR 20 response at week 24.
However, when you compare these numbers with the other TNF antagonists, ustekinumab does not appear to work as well in PsA. It remains to see how our rheumatology colleagues will regard this drug over time. It may be used as a second- or third-line drug for PsA.
3rd Generation Biologics
There is a down-stream effect from anti-IL-23 blockade feeding into IL-17 and down-regulating IL-22. Many pharmaceutical companies have been studying this pathway; there are two, possibly three, IL-23 inhibitors currently in trial, there are two anti-IL-17 inhibitors, an IL-17 receptor antagonist, and there was an IL-22 blocker that came and went…this is a very rich developmental pathway with many promises.
IL-17 Antagonists
The phase II studies of secukinumab (Novartis) a human IgG1 monoclonal IL-17A antibody demonstrated that the 150mg and 300mg doses were statistically significant as compared to placebo at week 12. This is an incredibly high-performance drug, i.e., in the 300mg dose, between 80 and 90 percent of these patients were achieving a PASI 75. According to Dr Leonardi, “this is absolutely remarkable when you think about the world of chronic inflammation”.
The results of secukinumab’s pivotal phase III data was released this fall at EADV. Dr. Leonardi reported that the ERASURE study evaluated secukinumab doses of 150 mg and 300 mg in a placebo controlled study. PASI-75 response rates in the secukinumab groups reached a peak at week 16 (80% – 90% range). IGA scores of “clear or almost clear” were reported in the 60% to 75% of patients. Treatment benefit was maintained through week 52 on q4 week injections following an induction period. Other phase 3 trials included the FIXTURE study included etanercept as an active control. In this comparator study patients showed a more rapid response to secukinumab vs etanercept (3 vs 8 wks) to achieve a PASI 50 and greater number of patients achieving PASI 90 (72.4% vs 41.5%). The third secukinumab Phase 3 trial SCULPTURE compared fixed-interval q4 weeks maintenance dosing with an “retreatment-as-needed” regimen. Data from follow-up to week 52 showed fixed interval therapy with SEC 300 mg and 150 mg q4wks sustained significantly greater PASI 75, 90 and 100 clearance rates over 1 year compared to “retreatment-as-needed”. “Retreatment as needed” is not a good regimen for any biologic”, says Dr. Leonardi.
We haven’t seen any of the safety results from the secukinumab phase III trials; however, the drug was well tolerated overall according to the phase II data according to Dr. Leonardi.
Ixekizumab (Lilly), is another anti-IL 17 monoclonal antibody, is another high-performance skin-clearing drug based upon its phase II data. Studies looking at 10, 25, 75 and 150 mg demonstrated that at the two highest doses, 80 percent of patients were at the PASI 75 range. There were no serious adverse events in this trial and the drug had a “remarkable safety profile” according to Dr. Leonardi.
The biostatisticians at Lilly, the makers of ixekizumab, utilized Youden’s Index to create a sensitivity and specificity assay. They determined that if they looked at the PASI 50 response at week four, they could make accurate predictions on the chances of success downstream. The likelihood of achieving PASI 75 and PASI 90 correlated with the PASI 50 response.
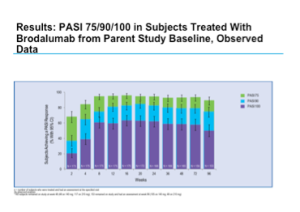
Brodalumab (Amgen) is an anti-IL-17 receptor antibody, which blocks not only Il-17A but also IL-17E and IL-17C. It is another high-performance drug for the treatment of psoriasis. The phase II studies, which were completed some time ago, demonstrated positive efficacy results along with a good safety profile. The long-term maintenance of clinical response with brodalumab has been demonstrated through week 96 of an open-label extension study. The one caveat with this open-label extension study is that it is an observed analysis, not an intent-to-treat approach to the data. Remember that the patients who came out of the trial are those who are not doing well so as you move through the trial, the population will continue to improve.
Positive results were also seen with sPGA over 96 weeks.
Interleukin 23
Remember that the p19 subunit is NOT shared; therefore, an anti-P19 molecule will block just IL-23. Tildrakizumab, a novel anti-IL23p19 monoclonal antibody, demonstrated good results in a 16-week phase IIB trial. There were four doses at weeks 0 and 4 (5mg, 25mg, 100mg, and 200mg) versus placebo. PASI 75 was achieved in 35, 65.5, 67.1, and 76.2 percent of patients, respectively versus 4.9 percent in the placebo arm and PASI 90 was achieved in 11.9, 24.4, 38.2, and 51.2 percent of patients versus 2.2 percent in the placebo arm. Tildrakizumab appears to be generally safe and well tolerated.
Small Molecules
Apremilast (Celgene)is a novel, small molecule that inhibits PDE-4. It has a variety of immunosuppressive effects, in that it reduces TNF-alpha, IL-2, IFN-γ and several leukotrienes. In Phase III studies (ESTEEM I and II), apremilast (30 mg BID) has achieved a PASI 75 rate of 33 percent. Importantly, scalp, nail and pruritus scores were superior in the apremilast patients compared to controls. Phase III results also demonstrated greater improvements from baseline Dermatology Quality of Life Index versus placebo. The majority of adverse events (AEs) were not serious and included mild gastrointestinal side effects: nausea 16% and diarrhea 19%.
According to Dr. Leonardi, apremilast also has phase III PsA results that are statistically significant versus placebo although not quite as robust as the TNF inhibitors. (FDA approval for apremilast for the treatment of PsA was announced during the winter AAD).
Tofacitinib (Pfizer), a novel, oral JAK inhibitor approved for the treatment of rheumatoid arthritis, is currently being investigated as a treatment for psoriasis among other indications. At week 12, PASI 75 response rates were significantly higher for all tofacitinib twice-daily groups: 25% (2mg), 40.8% (5 mg) and 66.7% (15 mg), compared with placebo (2%). It appears to work about as good as methotrexate. Of note, in rheumatoid arthritis, the FDA approved only the 5mg dose. The phase II safety data reported five serious adverse events (SAEs), three of which (atrial fibrillation, pyelnephritis, and urosepsis) occurred in one patient on the 2mg BID dose. There was a mild increase in Hgb levels that was greater in the 15mg BID group. There was a transient decrease in PMNs in the 5mg and 15mg BID groups and there were dose-related increases in both HDL and LDL. In the rheumatoid arthritis trials, the FDA found that the numerical increase in malignancy incidence over time was of particular concern and is consistent with a scenario where increasing exposure to tofacitinib increases the risk of malignancies. The FDA also stated that opportunistic infections, including TB, were “not uncommon.” In the RA program, 14 out of 15 patients who died due to infection were on tofacitinib. There were 34 patients with opportunistic infections, all of whom were on tofacitinib. Despite the immunological differences between rheumatoid arthritis and psoriasis patients, this is remarkable data and something that we, as dermatologists, need to pay particular attention to.
Conclusions
In conclusion, we can see an explosion of new biologics in the marketplace with most of the drugs targeting the IL-23 pathway. We still have an unresolved issue with regards to major adverse cardiovascular events (MACE). Some feel that there is a signal at the IL-12/23 blockade level, it will be interesting to see if the MACE signal populates the IL-17 blockers…there may be more information coming out on that. We are also seeing a wide variety of novel small molecules demonstrating moderate to robust efficacies and the safety profiles for some of these products are promising. There has also been a move to explore the JAK inhibitor in a topical formulation to treat psoriasis.
Psoriasis patients are now the “model” of choice for chronic inflammatory disease and we are getting these drugs earlier now than ever before. Remember that many of the drugs have fallen by the wayside for efficacy and/or safety issues. We need to select drugs that are good for our specialty and for our patients. As dermatologists, we need to consider that short- and long-term safety issues do exist; however, the benefit/risk ratio appears to be favorable overall.
Our treatment paradigm has shifted from a stratified approach, i.e., patients must fail the previous “step” of therapy before initiating a more “aggressive” therapy to an approach whereby the choice of therapy depends upon individual patient characteristics.
MauiDerm News Editor- Judy Seraphine